The DEMAND trial (NCT04224636) has successfully completed its enrollment, marking an important milestone in advancing the integration of immunotherapy and locoregional treatment for hepatocellular carcinoma (HCC). This investigator-initiated, multicenter, randomized Phase II study, sponsored by Ludwig-Maximilians-University of Munich and led by Dr. Enrico De Toni, explores two strategies combining atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) with transarterial chemoembolization (TACE) in patients with unresectable or intermediate-stage HCC.
Conducted across seven leading German academic centers—including Munich, Cologne, Bonn, Regensburg, Würzburg, and Tübingen—the DEMAND study is the first randomized trial to evaluate whether up-front atezolizumab + bevacizumab followed by on-demand selective TACE (sdTACE) or initial synchronous TACE with atezolizumab + bevacizumab can improve survival and local disease control in this patient population.
Background
Hepatocellular carcinoma (HCC) remains one of the leading causes of cancer-related mortality worldwide. Many patients are diagnosed at intermediate or advanced stages, when curative options such as resection or ablation are no longer feasible. For decades, transarterial chemoembolization (TACE) has been the standard of care for intermediate-stage HCC, achieving local tumor control by selectively delivering chemotherapy and embolic agents to tumor-feeding arteries. However, despite its widespread use, TACE alone often fails to achieve durable remission. Tumor recurrence, treatment resistance, and deterioration of liver function frequently limit long-term outcomes.
The advent of immunotherapy has profoundly altered the treatment landscape of HCC. The combination of atezolizumab (a monoclonal antibody targeting PD-L1) and bevacizumab (a monoclonal antibody targeting VEGF) demonstrated superior overall and progression-free survival compared with sorafenib in the landmark IMbrave150 trial, leading to its global approval as the first-line systemic standard for unresectable HCC. Beyond its systemic efficacy, this regimen has shown a favorable safety profile and a potential to downstage disease, supporting its exploration in earlier treatment settings.
From a biological standpoint, the rationale for combining immunotherapy with TACE is compelling. TACE induces tumor necrosis and ischemia, which can release tumor-associated antigens and promote immunogenic cell death, thereby priming the immune system. However, TACE also triggers hypoxia-induced VEGF upregulation, leading to angiogenesis and immune suppression within the tumor microenvironment. Bevacizumab counteracts this effect by inhibiting VEGF signaling, restoring vascular normalization and improving immune cell infiltration. Meanwhile, atezolizumab blocks PD-L1, preventing immune evasion by tumor cells. The combined effect is hypothesized to enhance both local and systemic tumor control, making immunotherapy-TACE integration an attractive therapeutic strategy.
The DEMAND trial was designed within this evolving clinical paradigm to systematically evaluate whether the sequential or synchronous combination of atezolizumab and bevacizumab with TACE can further improve survival in patients with intermediate or unresectable HCC. By doing so, it aims to generate evidence that bridges the current gap between locoregional and systemic therapies, defining how best to combine these modalities for maximum efficacy and safety.
Study Design
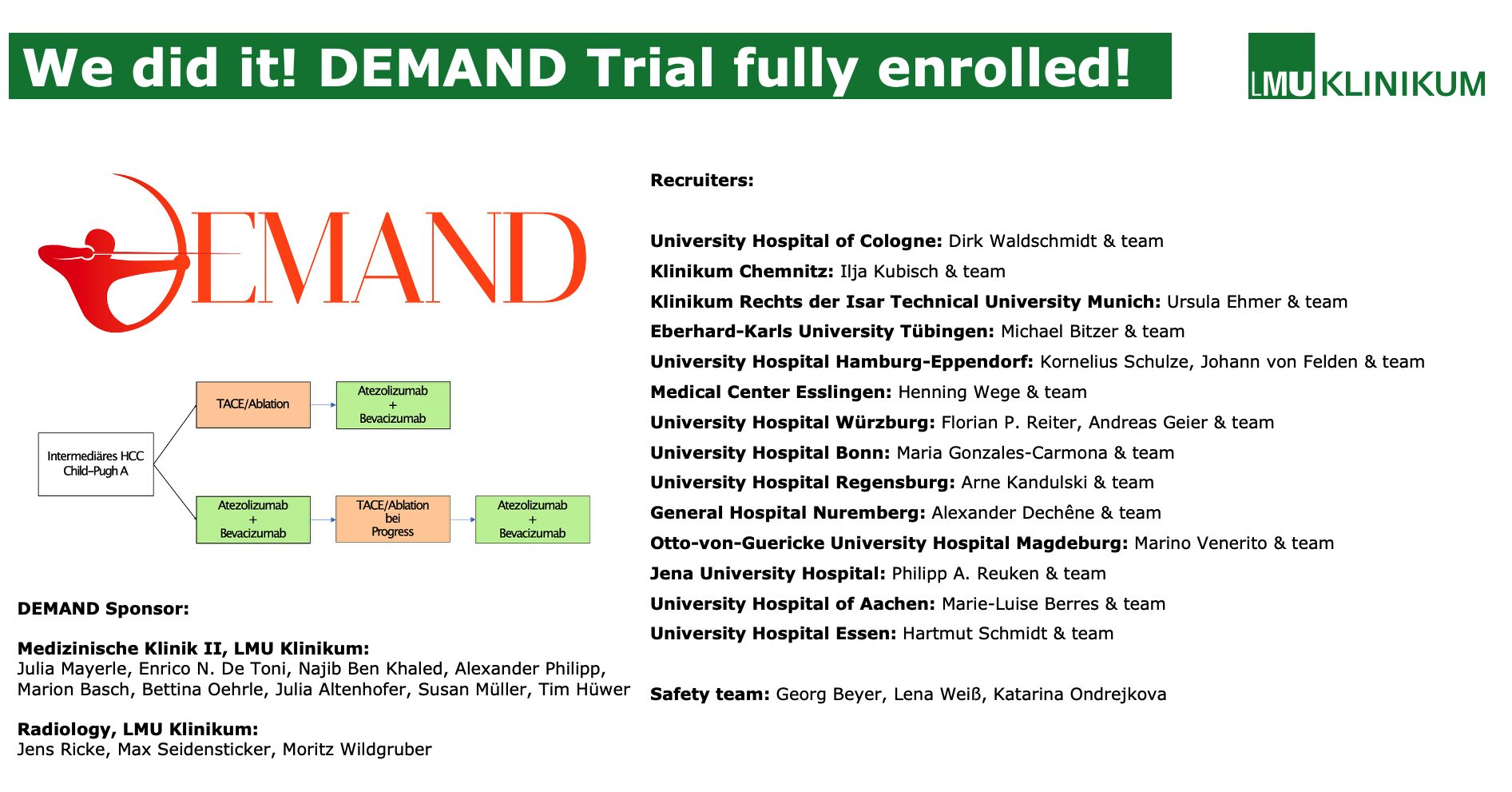
DEMAND is a randomized, two-arm, open-label Phase II trial enrolling 106 patients with histologically confirmed, non-resectable HCC suitable for TACE, ECOG 0–1, and Child-Pugh A–B7 liver function.
- Arm A: Patients receive atezolizumab + bevacizumab every three weeks for up to 24 months, with selective TACE performed only upon detection of unequivocal intrahepatic progression (sdTACE).
- Arm B: Patients undergo initial synchronous TACE targeting all viable lesions, followed within three days by atezolizumab + bevacizumab.
The primary endpoint is the 24-month survival rate, while secondary endpoints include overall response rate (ORR), progression-free survival (PFS), median overall survival (mOS), complete and disease control rates, time to untreatable progression, and quality-of-life assessments using EORTC QLQ-C30 and QLQ-HCC18 questionnaires.
Safety outcomes are being evaluated according to CTCAE v5.0.
Multidisciplinary Collaboration
The study’s success in achieving full enrollment reflects a strong multidisciplinary effort across German university hospitals, uniting experts in gastrointestinal oncology and interventional radiology.
Study management at LMU Klinikum was led by Dr. Bettina Oehrle and Dr. Marion Basch, with radiology oversight by Prof. Max Seidensticker. The steering committee includes Prof. Jens Ricke and Prof. Julia Mayerle, with additional support from Roche, which provided atezolizumab and bevacizumab and funded the study.
The trial’s progress was celebrated on World Cancer Research Day, emphasizing the collective dedication of patients, families, and clinical teams toward improving therapeutic options in HCC.
Clinical Perspective
By directly comparing sequential versus synchronous integration of atezolizumab + bevacizumab with TACE, the DEMAND trial aims to clarify how best to combine systemic immunotherapy with locoregional approaches in intermediate-stage disease. Results from DEMAND are expected to inform the design of future combination protocols and refine treatment sequencing for patients who fall between the conventional boundaries of locoregional and systemic therapy.
The study’s anticipated completion is March 2025, with outcomes likely to guide future Phase III investigations on optimizing multimodal management in hepatocellular carcinoma.
You can read more information about trial here.