BRAFV600E mutated non-small-cell lung cancer (NSCLC) represents a distinct molecular subtype that has traditionally been managed with targeted therapy using BRAF and MEK inhibitors, supported by early-phase trials demonstrating high objective response rates. However, as growing evidence links BRAFV600E mutations to smoking history and high PD-L1 expression, interest has shifted toward evaluating immune checkpoint inhibitors (ICIs) as a potential first-line option capable of delivering more durable long-term outcomes.
The FRONT-BRAF study, published in The Lancet Oncology (October 2025), is the largest multicenter analysis to date directly comparing first-line immunotherapy (with or without chemotherapy) versus BRAF/MEK inhibition in this rare NSCLC subtype. Its findings provide valuable real-world evidence that could reshape first-line treatment decisions for patients with BRAFV600E-driven lung cancer.
Title: First-line immunotherapy with or without chemotherapy versus BRAF plus MEK inhibitors in BRAFV600E-mutated metastatic non-small-cell lung cancer (FRONT-BRAF).
Authors: Alessandro Di Federico, MD, Kaiwen Wang, MD, Monica F Chen, MD, Adam A Barsouk, MD, Arianna Pagliaro, MD, Lanyi N Chen, MD, Francesca R Ogliari, MD, Paul Stockhammer, MD, Rajat Thawani, MD, Shahm Raslan, MD, Eleonora Gariazzo, MD, Francesca Fusco, MD, Grace M Hambelton, MD, Fabrizio Citarella, MD, David Meyer, MD, Mihaela Aldea, MD, Andrea De Giglio, MD, Joao V Alessi, MD, Federica Pecci, MD, Francesco Gelsomino, MD, Marcelo Corassa, MD, Natalie I Vokes, MD, Xinan Wang, PhD, Dario de Biase, PhD, Fawzi Abu Rous, MD, Phurin Areesawangkit, Omar Elghawy, Alessio Cortellini, MD, Giulio Metro, MD, Roberto Ferrara, MD, Mark M Awad, MD, Aliyah Pabani, MD, Joseph C Murray, MD, Federico Cappuzzo, MD, Prof Marina C Garassino, MD, Ibiayi Dagogo-Jack, MD, Prof Gregory Riely, MD, Michael Grant, MD, Benjamin O Herzberg, MD, Prof Andrea Ardizzoni, MD, Prof David Planchard, MD, Prof Bruce E Johnson, MD, Prof Corey J Langer, MD, Michael Offin, MDab, Marcelo V Negrao, MDd, Biagio Ricciuti, MD
Background
The BRAFV600E mutation occurs in approximately 1–3% of non-small-cell lung cancers (NSCLC) and defines a biologically distinct subset often associated with tobacco exposure and high PD-L1 expression. Unlike EGFR or ALK-driven tumors, BRAFV600E-mutated NSCLCs display greater immunogenicity, raising questions about the optimal first-line systemic therapy.
Current treatment guidelines by NCCN and ESMO favor BRAF plus MEK inhibitors—such as dabrafenib plus trametinib or encorafenib plus binimetinib—as the preferred initial regimen, based on early phase II data showing response rates over 60% and median progression-free survival (PFS) of 10–12 months. However, immune checkpoint inhibitors (ICIs) have shown substantial benefit in retrospective series, particularly in tumors with high PD-L1 and smoking history.
The FRONT-BRAF study, published in The Lancet Oncology (October 2025), provides the largest multicenter, real-world comparison to date between first-line ICI-based therapy (with or without chemotherapy) and BRAF/MEK targeted therapy in metastatic BRAFV600E-mutated NSCLC.
Methods
This retrospective cohort study was conducted across 17 centers in the USA, Italy, France, and Brazil, enrolling adults aged ≥18 years with stage IV, treatment-naive, BRAFV600E-mutated NSCLC and ECOG performance status 0–3. Eligible patients received either:
- Immune checkpoint inhibitors (ICIs) – PD-1 or PD-L1 inhibitors, with or without platinum-based chemotherapy
- BRAF and MEK inhibitors – dabrafenib plus trametinib, or encorafenib plus binimetinib.
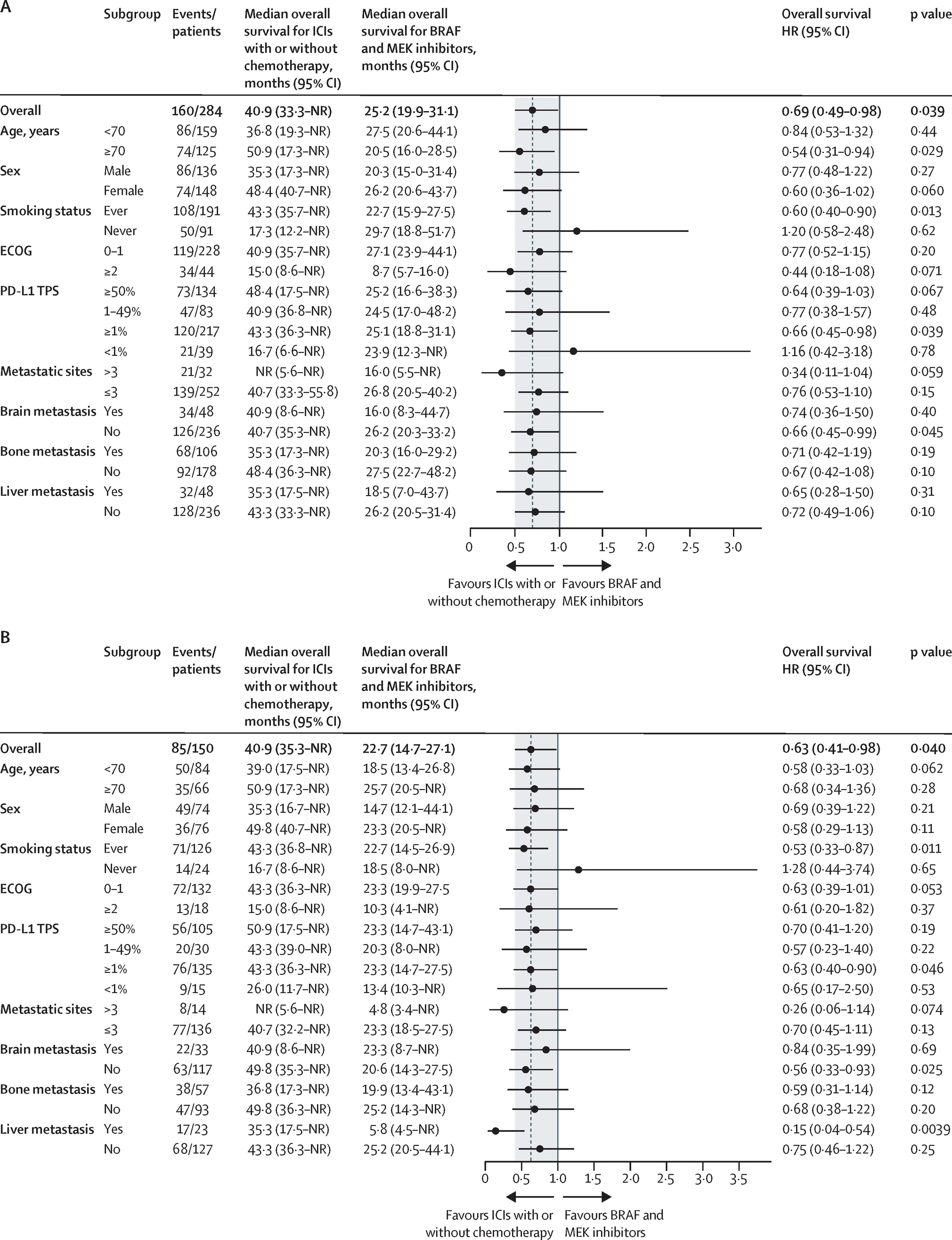
The primary endpoint was overall survival (OS). Secondary endpoints included PFS, objective response rate (ORR), and exploratory subgroup analyses by clinical and molecular characteristics, including PD-L1 expression, smoking history, presence of brain metastases, and co-mutations in genes such as TP53, PIK3CA, IDH1, and SETD2.
Propensity-score matching and multivariable Cox regression models were used to adjust for baseline imbalances.
Study Design and Population
A total of 284 patients were included:
- 88 (31%) received first-line ICIs ± chemotherapy
- 196 (69%) received first-line BRAF/MEK inhibitors.
The median age was 68 years (IQR 61–74), with 52% female.Patients treated with ICIs were more likely to have a history of smoking (83% vs 60%) and higher PD-L1 expression (≥50% in 66% vs 39%) than those receiving targeted therapy.Most participants (over 80%) had ECOG 0–1 and adenocarcinoma histology (≈95%).
Results
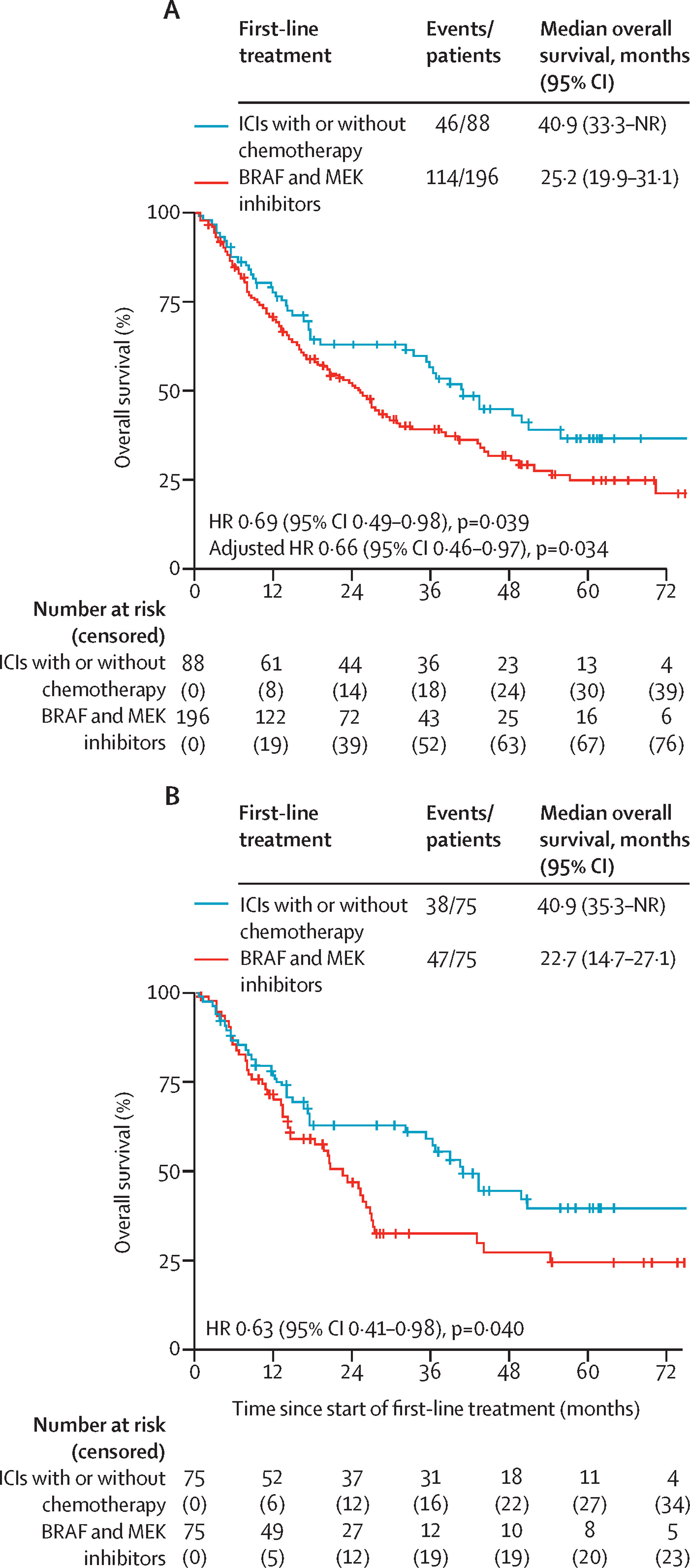
At a median follow-up of 45 months, first-line ICIs ± chemotherapy significantly improved survival outcomes compared with BRAF/MEK inhibitors:
Median Overall Survival (OS):
- 40.9 months (95% CI 33.3–not reached) with ICIs ± chemotherapy
- 25.2 months (19.9–31.1) with BRAF/MEK inhibitors
- Hazard ratio (HR) = 0.69 (95% CI 0.49–0.98, p=0.039)
This survival advantage persisted after adjustment for confounders (multivariate HR 0.66, p=0.034) and was confirmed in a propensity-matched cohort (HR 0.63, p=0.040).
Subgroup benefits with ICIs ± chemotherapy were most evident in:
- Patients aged ≥70 years (HR 0.54, p=0.029)
- Smokers (HR 0.60, p=0.013)
- Tumors with PD-L1 ≥1% (HR 0.66, p=0.039)
- TP53 co-mutations (HR 0.46, p=0.0048)
- No brain metastases (HR 0.66, p=0.045)
Progression-Free Survival (PFS) was similar between both strategies, while objective response rates favored BRAF/MEK inhibitors:
ORR was higher with targeted therapy, consistent with the rapid cytoreductive effect of oncogene blockade.However, PFS2 and post-progression survival were longer in patients who started with ICIs, suggesting more durable disease control after immunotherapy.
Among 110 patients who received both therapies sequentially, the order of treatment did not significantly affect total survival, though first-line ICI use was associated with better post-progression outcomes.
Key Findings
- First-line immunotherapy (± chemotherapy) achieved longer median OS than BRAF/MEK inhibitors despite similar PFS.
- Targeted therapy offered higher ORR, confirming strong initial disease control but shorter durability.
- The benefit of immunotherapy was especially clear in smokers, PD-L1–positive tumors, and those with TP53 co-mutations—molecular features linked to higher tumor mutational burden and immune responsiveness.
- Patients without brain metastases derived the most survival advantage from ICIs, while those with CNS disease appeared to benefit equally or slightly more from targeted therapy, possibly due to better intracranial activity.
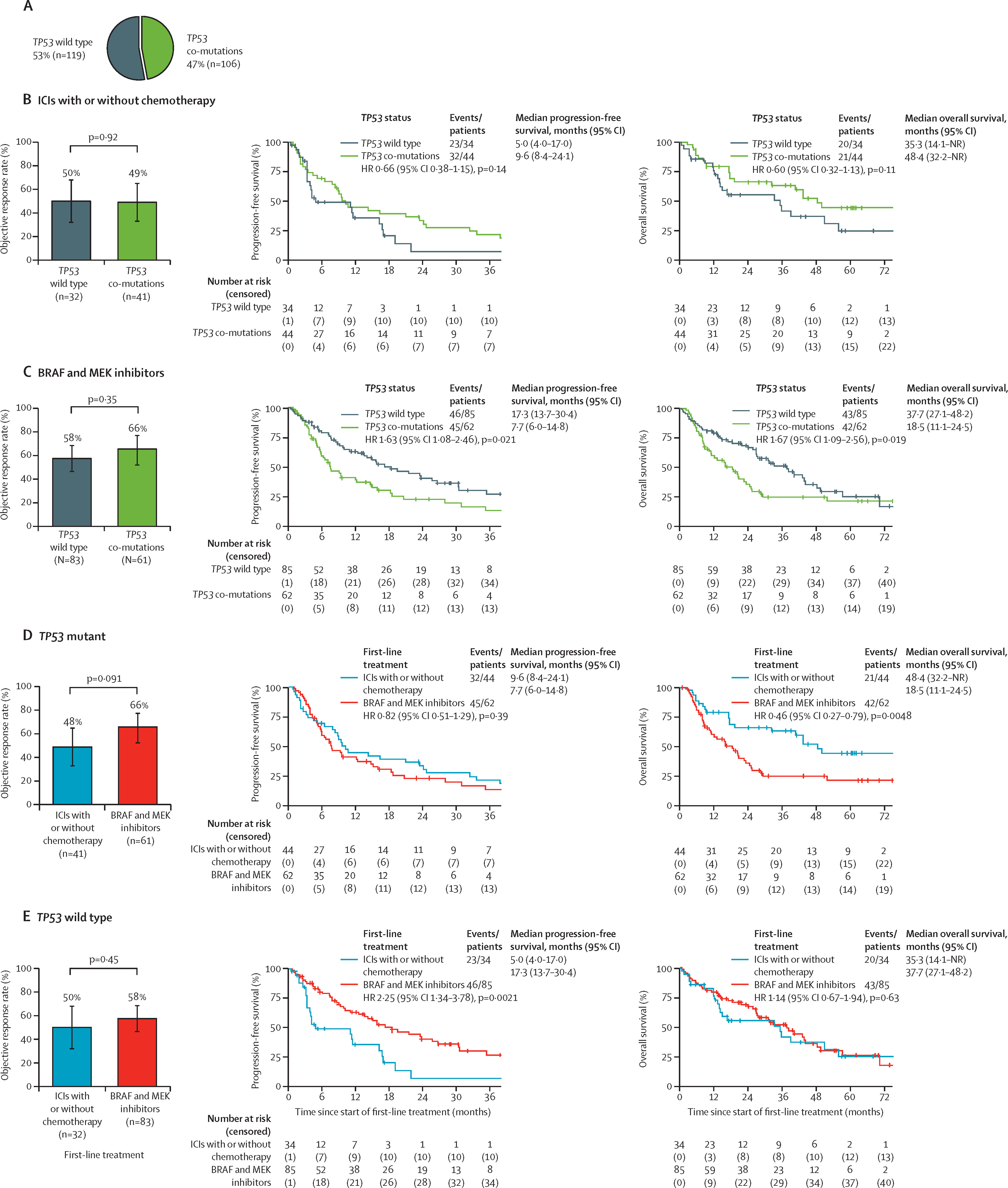
Co-mutation analyses provided additional insight:
- TP53 mutations predicted poor outcomes with BRAF/MEK inhibitors but favorable response to ICIs.
- PIK3CA co-mutations conferred worse PFS with ICIs but not with targeted therapy.
- IDH1 co-mutations were rare but associated with shorter PFS under BRAF/MEK inhibition.
- SETD2 co-mutations correlated with improved outcomes under targeted therapy, whereas SETD2 wild-type tumors favored ICIs.
Importantly, BRAF/MEK inhibitors administered after ICIs showed comparable safety to their use as first-line treatment, including no increase in pneumonitis or hepatic toxicity, alleviating prior concerns about sequential use.
Key Takeaway Messages
The FRONT-BRAF study challenges current treatment paradigms for BRAFV600E-mutated metastatic NSCLC.While targeted therapy remains effective for tumor shrinkage, first-line ICIs ± chemotherapy deliver a clinically meaningful survival advantage (median OS ~41 months vs 25 months), particularly in patients with immunogenic tumor profiles (smokers, PD-L1 ≥1%, TP53 co-mutations, no brain metastases).
These findings parallel evidence from melanoma trials (DREAMseq, SECOMBIT) that established immunotherapy first, targeted therapy later as a superior strategy for BRAFV600E-mutated melanoma.
Importantly, BRAF/MEK inhibitors remain a valid second-line option, offering high response rates without compromising safety after prior ICI exposure.
Conclusion
The multicenter FRONT-BRAF study provides compelling evidence that immunotherapy-based regimens (with or without chemotherapy) should be considered as a preferred first-line treatment for metastatic BRAFV600E-mutated NSCLC, particularly in patients with favorable immune-related features.
Although retrospective in design, the study’s robust analysis and international cohort support a shift toward individualized, biomarker-guided therapy selection, integrating PD-L1 status, smoking history, and co-mutational landscape into decision making.Future prospective randomized trials are needed to confirm these findings and refine the optimal sequencing of immunotherapy and targeted therapy in this rare but clinically significant NSCLC subgroup.
You can read the full artcle here.


