SBRT (Stereotactic body radiotherapy) has emerged as an established treatment option for localized prostate cancer, supported by the tumor’s low α/β ratio and advances in image-guided radiotherapy. In a Spanish multicenter cohort of 250 patients treated with 36.25–40 Gy in five fractions, investigators evaluated 2-year biochemical control, radiological progression, survival, and late toxicity to assess the real-world effectiveness and safety of prostate SBRT.
Title: Biochemical Control and 2‑year Tolerability Following Stereotactic Body Radiotherapy for Localized Prostate Cancer: A Multicenter Study in Spain
Authors: SigfredoEliasRomeroZoghbi, FernandoLópezCampos, AbrahamsOcanto, DavidSanz‑Rosa, IsraelJohnThuissard‑Vasallo, CristinaAndreuVázquez, CristinaLaria, JaumeFernándezIbiza, JonAndreescuYagüe, EvitaKrumina, MariaMateos, MaiaDzhugashvili, LuisAlbertoGlaría, DanielaGonsalves, CastaliaFernández, DavidEsteban, JoséBegaradelaFuente, JoséAntonioGonzálezFerreira, AntonioRistori, DanielRivas, EscarlataLópez, AnaBelénBezaresAlarcón, LoubnaAakki, LuisLarrea, JoséÁngelGarcíaCuesta, ConstantinosZamboglou, FilippoAlongi, RafaelGarcíaGarcía, FelipeCouñago
Background
Stereotactic body radiotherapy (SBRT) has become a major option for localized prostate cancer because prostate tumors are thought to be particularly sensitive to larger doses per fraction, while modern image guidance and motion management allow high-dose precision with short treatment courses. Clinical trials and large institutional series have reported strong biochemical control with acceptable toxicity, but multicenter “routine practice” outcomes remain important because real-world populations include older patients, common comorbidities, variable platforms, and individualized use of androgen deprivation therapy (ADT).
This Spanish multicenter study evaluated early oncologic outcomes and late adverse events at 2 years after SBRT for localized prostate cancer across 12 centers, building on the group’s prior work in the same network on acute toxicity.
Methods
Investigators analyzed men treated with SBRT for histologically confirmed localized prostate adenocarcinoma across 12 Spanish centers between January 2020 and December 2023. The primary endpoint was biochemical recurrence-free survival (bRFS), with biochemical recurrence defined by the Phoenix definition (PSA nadir + 2 ng/mL). Secondary endpoints included radiological progression-free survival (rPFS), overall survival (OS), and cancer-specific survival (CSS).
Late adverse events were evaluated between 6 and 24 months and graded using CTCAE v5.0, focusing on genitourinary (GU), gastrointestinal (GI), and sexual toxicity (erectile dysfunction). Follow-up included serial PSA measurements and testosterone monitoring to 24 months. Survival outcomes were estimated with Kaplan–Meier methodology and compared using log-rank testing. Logistic regression explored factors associated with grade ≥2 adverse events and recurrence, with clinically relevant variables and those meeting p<0.10 in univariate testing considered for multivariable models. Statistical analyses were performed using SPSS (version 29.0) with significance set at p<0.05.
Study Design
This was a retrospective, multicenter cohort study approved by a Drug Research Ethics Committee (approval no. 5585), using electronic medical records to capture baseline characteristics, treatment details, PSA kinetics, imaging-defined progression, and late toxicity outcomes. Eligible patients were men ≥18 years with localized prostate adenocarcinoma across all NCCN risk groups.
Eligibility criteria reflected SBRT practice standards. Prostate volume had to be ≤90 cm³, and patients with severe baseline urinary symptoms (IPSS >19) were excluded. A pelvic MRI within 6 months prior to SBRT was mandatory. High- and very high-risk patients underwent CT and bone scintigraphy for staging, as PSMA-PET was not routinely available. Patients with cN1 or M1 disease, or prior prostate radiotherapy or focal therapy, were excluded. Fiducial markers were placed for image guidance, and rectal spacers (Barrigel or SpaceOAR) were used in most patients. Prior TURP was allowed if performed ≥3 months before treatment.
ADT was prescribed according to risk group: ≤6 months for unfavorable intermediate-risk and 24–36 months for high/very high-risk disease. In selected intermediate-risk cases, ADT was omitted based on comorbidity, age, patient preference, or favorable tumor features. No androgen receptor inhibitors were used.
SBRT was delivered on Elekta, Varian, Accuray platforms (mostly conventional LINACs) using 36.25–40 Gy in five alternate-day fractions. For intermediate- and high-risk patients, 40 Gy was prescribed to D95% of the CTV with at least 36.25 Gy to 95% of the PTV; low-risk patients received 36.25 Gy. Planning followed ICRU 91 criteria (PTV D2% ≤110%, V100% ≥95%), with daily fiducial-based IGRT and intrafraction motion management. PTV margins were 5 mm (3 mm posteriorly). The urethra was contoured with a 0–2 mm PRV margin, and no formal dose constraints were applied to sexual function–related structures.
Results
A total of 250 patients were included (one excluded due to missing post-treatment PSA from an initial 251-patient cohort). Median follow-up was 24 months. Median age was 72 years (IQR 65–76) and median baseline PSA was 6.7 ng/mL (IQR 5.3–8.7). By NCCN risk group, the cohort consisted of 4.0% very low risk, 26.4% low risk, 26.8% favorable intermediate risk, 39.6% unfavorable intermediate risk, 2.8% high risk, and 0.4% very high risk. In practical terms, nearly two-thirds were intermediate risk (67% when combining favorable and unfavorable intermediate groups), with a smaller high/very high-risk fraction (3.2%). Baseline urinary symptoms were common but controlled: 56.0% had mild IPSS (0–7) and 44.0% had IPSS ≥8 (moderate or worse), while severe symptoms were excluded by design.
Treatment delivery reflected contemporary prostate SBRT practice. The median prescribed dose was 40 Gy in five fractions (IQR 36.25–40.0), delivered on alternate days. Median prostate volume was 54.0 cm³ (IQR 40.0–70.5) and the treated volume (prostate ± seminal vesicles) was 84.0 cm³ (IQR 59.0–113.0), with a maximum treated volume reaching 241 cm³. Rectal spacers were used in 99.2% of patients: Barrigel in 58.4%, SpaceOAR in 40.8%, and only two patients (0.8%) had no spacer. ADT was omitted in 66.5% of cases; 30.3% received short-term ADT (≤6 months) and 3.2% received long-term ADT (>6 months), consistent with the cohort’s risk distribution. Comorbidity was typical for a 72-year median-age population: 14.8% had diabetes, 48.0% hypertension, 22.0% prior cardiovascular events, and 19.2% were taking anticoagulant or antiplatelet therapy.
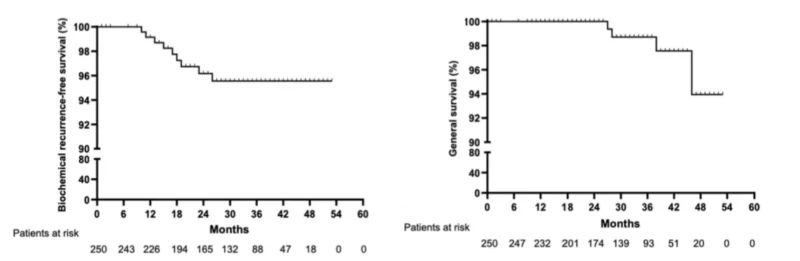
Oncologic outcomes at 2 years were strong. Biochemical recurrence occurred in 9 of 247 evaluable patients (3.6%), translating to a Kaplan–Meier estimated bRFS of 96.4% at 24 months. Recurrences clustered in higher-risk biology: 88.9% (8/9) occurred in the unfavorable intermediate-risk group and 11.1% (1/9) in the high-risk group, and all had Gleason scores ≥4+3 (ISUP grade group ≥3). Time to biochemical recurrence ranged from 11 to 22 months, with a median of 15 months. Radiological progression was uncommon and largely nodal: one local intraprostatic recurrence (0.4%), four pelvic nodal progressions (1.6%), and two extrapelvic progressions (0.8%). Notably, no bone or visceral metastases were observed within follow-up. At 24 months, radiological progression-free survival (rPFS) was 97.2%.
Survival outcomes were reassuring and aligned with early-stage disease expectations. At 24 months, overall survival was 95.6% (11 of 249 deaths), and none of the deaths were prostate cancer–related, yielding a cancer-specific survival of 100% at 2 years.
Late toxicity results are a key contribution because multicenter “real-world” tolerance is often questioned. The incidence of grade ≥2 late adverse events at 24 months was 7.6% in the GU domain, 1.2% in the GI domain, and 14.3% in the sexual domain among evaluable patients. GU events were dominated by low-grade symptoms such as grade 1 dysuria (20.6%). Grade ≥2 hematuria occurred in 2.0%, and grade 2 urinary incontinence was rare (0.4%). The study reports symptom resolution in 89.8% of cases, supporting that most late urinary effects were manageable and transient. GI toxicity was uncommon: grade 1 diarrhea or rectal bleeding occurred in 1.6%, and grade ≥2 GI events occurred in 1.2%, all involving rectal bleeding (including grade 3 events), with 97.9% free of GI symptoms at the end of follow-up.
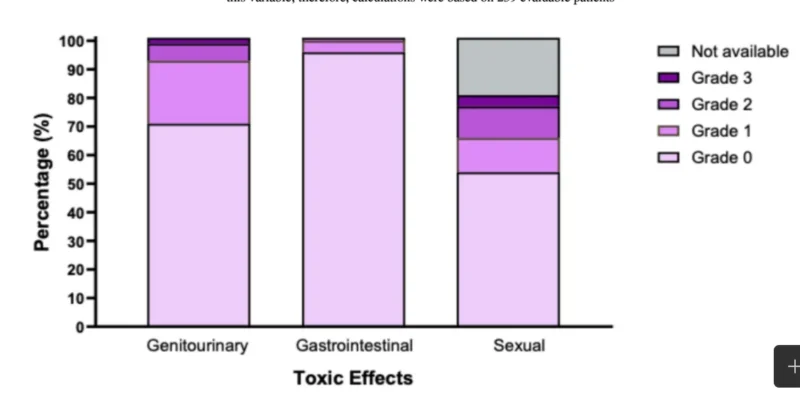
For sexual outcomes, among 239 evaluable patients, grade 1 erectile dysfunction occurred in 10.8%, and grade ≥2 erectile dysfunction occurred in 14.3% (grade 2 in 10.5% and grade 3 in 3.8%). By the end of follow-up, 77.5% were reported to have maintained or recovered erectile function, though interpretation should consider the absence of prospective dose constraints for sexual structures and typical limitations of retrospective sexual function documentation.
The exploratory modeling highlighted clinically meaningful associations. Diabetes mellitus was associated with higher risk of grade ≥2 GU toxicity and remained independently predictive in multivariable analysis: chronic urinary grade ≥2 events occurred in 16.2% of diabetics versus 3.4% of non-diabetics, with an adjusted odds ratio of 6.10 (95% CI 1.49–24.96; p=0.012). Interestingly, prescription of 40 Gy including the seminal vesicles appeared protective for GU grade ≥2 events (2.0% vs 8.1%; OR 0.20; 95% CI 0.06–0.74; p=0.016). Anticoagulant/antiplatelet therapy was not significantly associated with GU or sexual grade ≥2 adverse events (GU OR 2.91; p=0.057; sexual OR 1.40; p=0.622), and GI events were too few for robust modeling.
Key Findings
This 12-center Spanish real-world cohort demonstrates that prostate SBRT delivered as 36.25–40 Gy in five alternate-day fractions achieved a 2-year biochemical control rate of 96.4%, with biochemical recurrence limited to 3.6% and concentrated in patients with unfavorable intermediate-risk/high-risk features and ISUP ≥3. Radiological progression was rare (2.8%) and predominantly nodal, while 2-year cancer-specific survival was 100%. Late toxicity remained low—particularly GI toxicity (grade ≥2: 1.2%)—in a population where rectal spacers were used in 99.2% of patients. Diabetes emerged as a strong predictor of grade ≥2 late GU toxicity (adjusted OR 6.10), highlighting the need for careful baseline risk assessment in patient selection and counseling.
Conclusion
In a multicenter Spanish network experience including 250 men treated between 2020 and 2023, SBRT for localized prostate cancer (36.25–40 Gy in five fractions) produced high 2-year biochemical control (96.4%), minimal radiologic progression (2.8%), and excellent cancer-specific survival (100%) with low late toxicity (GU grade ≥2: 7.6%; GI grade ≥2: 1.2%; sexual grade ≥2: 14.3% in evaluable patients). These findings support SBRT as an effective and well-tolerated approach in routine clinical practice, particularly for carefully selected patients with controlled baseline urinary function and contemporary image-guided workflows. Longer follow-up will be essential to confirm durability beyond 2 years, clarify late sexual outcomes, and better define patterns of recurrence in higher-risk subgroups.


