The CHRYSALIS-2 trial prospectively evaluated the combination of amivantamab and lazertinib in patients with advanced non–small cell lung cancer (NSCLC) harboring atypical EGFR mutations—an uncommon but clinically challenging group that accounts for only 5%–10% of EGFR-mutated cases yet responds poorly to standard EGFR-TKIs.
Mutations such as G719X, L861Q, and S768I often exhibit heterogeneous drug sensitivity, resulting in shorter survival and limited targeted treatment options. While amivantamab and lazertinib have shown strong efficacy in common EGFR mutations, their role in atypical alterations has remained unclear.
Cohort C of this multi-cohort study was specifically designed to generate the first dedicated, prospective dataset in this population, excluding tumors with Ex19del, L858R, or exon 20 insertions to accurately characterize treatment benefit.
Title: Amivantamab Plus Lazertinib in Atypical EGFR-Mutated Advanced Non–Small Cell Lung Cancer: Results From CHRYSALIS-2
Authors: Pascale Tomasini, MD, MSc, Yongsheng Wang, MD, Yongsheng Li, MD, PhD, Enriqueta Felip, MD, PhD , Lin Wu, MD, Jiuwei Cui, MD, PhD, Benjamin Besse, MD, PhD, Alexander I. Spira, MD, PhD , Joel W. Neal, MD, PhD , Koichi Goto, MD, PhD , Christina S. Baik, MD, MPH, Melina E. Marmarelis, MD , Eiki Ichihara, MD, Yiping Zhang, MD, Jong-Seok Lee, MD, PhD, Se-Hoon Lee, MD, PhD,
James Chih-Hsin Yang, MD, PhD, Sebastian Michels, MD, Zacharias Anastasiou, MS, Joshua C. Curtin, PhD, Xuesong Lyu, PhD, Janine Mahoney, BSN, RN, Levon Demirdjian, PhD, Craig S. Meyer, MS, PhD, MPH, Youyi Zhang, PhD, Isabelle Leconte, PhD, Patricia Lorenzini, MS, Roland E. Knoblauch, MD, PhD, Leonardo Trani, MD, Mahadi Baig, MD, Joshua M. Bauml, MD and Byoung Chul Cho, MD, PhD
Published in JCO, December 2025
Background
Non–small cell lung cancer (NSCLC) accounts for around 80%–85% of lung cancer cases and is biologically heterogeneous. Among patients with EGFR-mutated NSCLC, most harbor common alterations such as exon 19 deletions (Ex19del) or exon 21 L858R, which represent about 85%–90% of EGFR mutations. Atypical (uncommon) EGFR mutations and exon 20 insertions each account for roughly 5%–10% of EGFR-mutated NSCLC.
The most frequent atypical mutations include G719X (exon 18), S768I (exon 20) and L861Q (exon 21), which may occur as solitary or compound alterations.
Outcomes for patients with atypical EGFR mutations are significantly worse than for those with common mutations when treated with EGFR tyrosine kinase inhibitors (TKIs).
Survival is particularly poor when atypical mutations are present in compound form without accompanying common mutations. Afatinib is currently approved for certain nonresistant atypical EGFR mutations (G719X, S768I, L861Q), and osimertinib is widely used off-label in this setting, but progression-free survival (PFS) generally remains under one year and benefit is variable across mutation subtypes. There is a clear need for more effective, biomarker-directed therapies for this rare but clinically important group.
Amivantamab is a bispecific antibody targeting EGFR and MET, with multiple mechanisms including ligand blockade, receptor internalization and immune cell engagement. Lazertinib is a CNS-penetrant, third-generation EGFR-TKI. The combination has demonstrated superior efficacy versus osimertinib in common EGFR-mutated NSCLC (MARIPOSA trial) and is now approved as first-line therapy for Ex19del or L858R. Cohort C of the CHRYSALIS-2 study prospectively evaluated amivantamab plus lazertinib in patients with advanced NSCLC harboring atypical EGFR mutations, excluding tumors with coexisting common EGFR driver mutations.
Methods and Study Design
CHRYSALIS-2 is a multi-cohort, phase I/Ib study evaluating lazertinib alone or in combination with amivantamab across several distinct clinical settings of EGFR-mutated NSCLC. Each cohort was designed to answer a separate biological and therapeutic question—for example, some cohorts focused on patients who had progressed after osimertinib, while others explored specific resistance mechanisms or prior treatment pathways.
Cohort C represents only one component of the overall CHRYSALIS-2 program, dedicated specifically to patients with atypical EGFR mutations such as G719X, L861Q, and S768I. Importantly, this cohort prospectively excluded tumors with coexisting common EGFR mutations (Ex19del, L858R) or exon 20 insertions, allowing a clean evaluation of amivantamab-lazertinib efficacy in a population traditionally underserved by standard EGFR-TKIs. Results presented in this article therefore reflect the outcomes from Cohort C alone, not from the entire CHRYSALIS-2 study. Eligible patients had:
- Atypical EGFR mutations without Ex19del, L858R, or exon 20 insertions, either as solitary or compound atypical mutations.
- Up to two prior lines of systemic therapy (chemotherapy and/or first- or second-generation EGFR-TKIs).
- No prior third-generation EGFR-TKI exposure.
- Patients could be treatment-naïve or previously treated. Those with stable, treated CNS metastases were allowed.
The primary endpoint was investigator-assessed objective response rate (ORR) per RECIST v1.1. Secondary endpoints included duration of response (DoR), clinical benefit rate (CBR), PFS, overall survival (OS), time to treatment discontinuation and safety. Exploratory endpoints included circulating tumor DNA (ctDNA) profiling, assessment of TP53 co-mutations and structural classification of EGFR mutations (P-loop and αC-helix compressing [PACC], classical-like, T790M-like).
Treatment was given in 28-day cycles until disease progression, unacceptable toxicity, withdrawal, noncompliance or investigator decision.
- Lazertinib: 240 mg orally once daily.
- Amivantamab: Intravenous administration once weekly in Cycle 1 and then every 2 weeks thereafter. Dosing was weight-based-1,050 mg for patients <80 kg, 1,400 mg for patients ≥80 kg. The first dose was split over two days (350 mg on Cycle 1 Day 1 and the remainder on Day 2) to mitigate infusion-related reactions.
Results
As of January 12, 2024, 105 participants received amivantamab-lazertinib in Cohort C 49 (47%) were treatment-naïve56 (53%) had received prior systemic therapy (88% prior EGFR-TKI, predominantly afatinib). Key baseline characteristics were following-median age: 64 years (range 30–85), sex: 50% male, race: 68% Asian, 30% White.
The most common atypical EGFR mutation patterns included G719X in 56% of patients, L861X in 26%, and S768X in 23%, occurring as either single or compound alterations; overall, 28% of patients harbored compound atypical mutations. Brain or CNS metastases were present at baseline in 35% of the cohort.ECOG performance status was 0 in 31% and 1 in 69% of the cohort.
At a median follow-up of 16.1 months, median treatment duration was 11.1 months. In the overall population:
- ORR: 52% (all partial responses; 95% CI, 42–62)
- Median DoR: 14.1 months (95% CI, 9.5–26.2)
- CBR: 79% (95% CI, 70–86)
- Median PFS: 11.1 months (95% CI, 7.8–17.8)
- Median OS: not estimable (95% CI, 22.8 to NE)
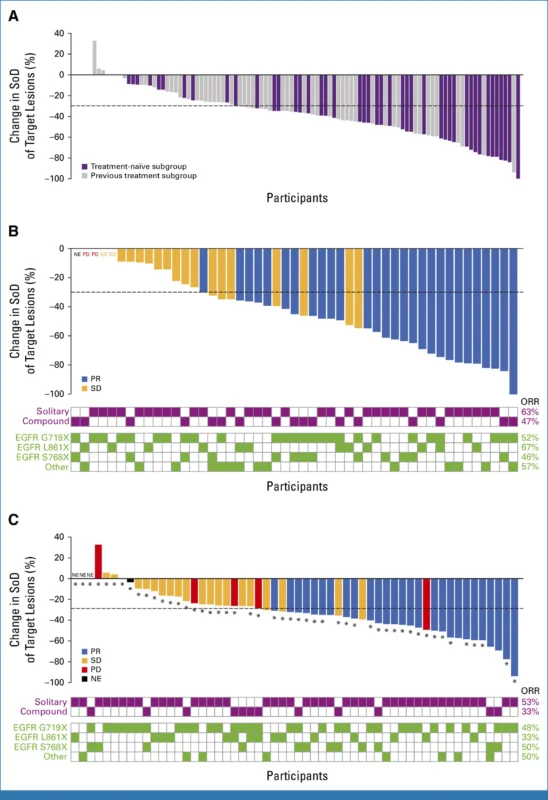
Treatment-naïve subgroup-among 49 first-line patients, with a median follow-up of 17.3 months:
- ORR: 57% (95% CI, 42–71)
- Median DoR: 20.7 months (95% CI, 9.9 to NE)
- CBR: 84% (95% CI, 70–93)
- Median PFS: 19.5 months (95% CI, 11.2 to NE)
- Median OS: not estimable (95% CI, 26.3 to NE)
- 24-month OS rate: 77%
These PFS and OS outcomes compare favorably with historical data for afatinib and osimertinib in atypical EGFR-mutated NSCLC, where PFS has generally been reported under 12 months.
Previously treated subgroup- in 56 patients who had received prior therapy, median follow-up was 15.4 months:
- ORR: 48% (95% CI, 35–62)
- Median DoR: 11.0 months (95% CI, 4.5 to NE)
- CBR: 75% (95% CI, 62–86)
- Median PFS: 7.8 months (95% CI, 5.4–11.1)
- Median OS: 22.8 months (95% CI, 16.9 to NE)
Key findings of CHRYSALIS-2 Trial
Amivantamab-lazertinib demonstrated robust activity across a spectrum of atypical EGFR mutations, including both solitary and compound alterations. Solitary versus compound status did not meaningfully alter ORR; 95% confidence intervals for response overlapped in both treatment-naïve and pretreated settings.
Structural classification of EGFR mutations (per Robichaux et al.) showed:
- PACC mutations: ORR 45% (n = 38)
- Classical-like mutations: ORR 64% (n = 14)
- T790M-like mutations: ORR 67% (n = 3)
This indicates clinically relevant activity across different structural mutation classes, including those traditionally considered more difficult to target with TKIs.
Among patients with analyzable baseline ctDNA, TP53 co-mutations were present in 63%. Importantly, ORR remained high regardless of TP53 status: 54% (95% CI, 37–71) with TP53 mutations versus 46% (95% CI, 28–66) without, suggesting that TP53 co-mutation did not preclude benefit from the combination.
Conclusion
CHRYSALIS-2 Cohort C represents the largest prospective, single-cohort study to date focusing on atypical EGFR-mutated advanced NSCLC without coexisting common EGFR driver mutations. In this challenging setting, amivantamab plus lazertinib produced high response rates, long response durations and encouraging progression-free and overall survival, particularly in the first-line setting.
The combination demonstrated activity across diverse atypical EGFR mutations and structural classes, and its efficacy appeared preserved in patients with compound mutations and TP53 co-mutations. Safety findings were consistent with previous amivantamab-lazertinib experience, and no new safety signals emerged.
Taken together, these results support amivantamab-lazertinib as a promising treatment option for patients with advanced NSCLC harboring atypical EGFR mutations and strengthen the role of dual EGFR/MET targeting combined with third-generation EGFR-TKI therapy in expanding precision medicine beyond the common Ex19del and L858R alterations.
You can read the full article here.