Amol Akhade, Senior Consultant at Fortis Hospitals Mumbai, highlighted emerging data and clinical insights from WCLC25 in lung cancer.
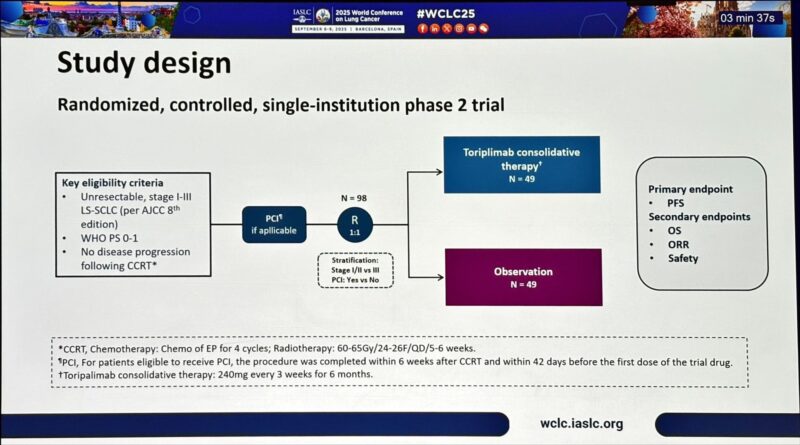
“Toripalimab consolidation after CCRT in LS-SCLC at WCLC25
Small, single-institution phase II (N=98) reports impressive outcomes:
- PFS HR 0.54 (NR vs 14m, p=0.03)
- OS HR 0.41 (NR vs 30m, p=0.01)
- ↓ brain progression (HR 0.47)
- 2y OS ~79% vs 59%.
At first glance, outcomes look stronger than ADRIATIC (durvalumab, phase III, N=749, global) where:
- PFS HR 0.76
- OS HR 0.70 (56 vs 33m)
- Brain relapse HR 0.64
But context matters
- Design: Toripalimab = single center, phase II, only 98 patients, 6m of IO. ADRIATIC = global, phase III, 749 pts, 24m IO.
- Population: 100% Asian vs 50% Asian, 50% rest of world in ADRIATIC.
- Endpoints: Toripalimab = PFS primary; ADRIATIC = OS and PFS co-primary.
- PCI effect: OS benefit stronger in those without PCI → raises question if CNS protection is the real driver.
- Follow-up: OS NR, risk of attenuation with maturity.
Critical view:
Signal is promising, especially for CNS outcomes.
But small, immature trial → likely overestimation of effect sizes.
ADRIATIC remains definitive standard.
Key unresolved question: is 6 months of IO enough, or is 24 months needed for durable control?
For now → Toripalimab may be a potential LMIC option (shorter, cheaper), but only phase III validation will tell if ‘less is more.’”
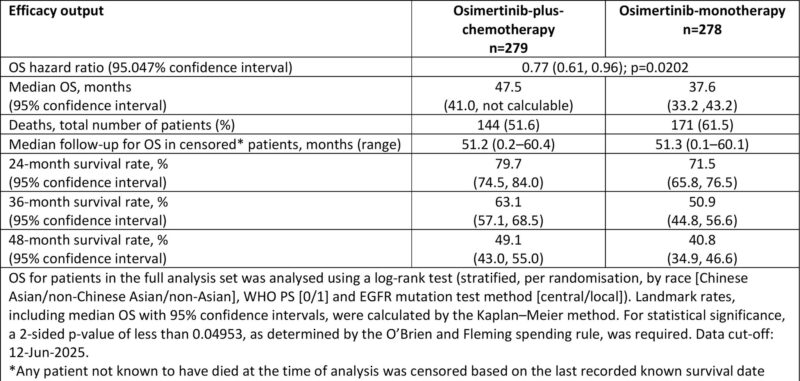
- 1L EGFRm NSCLC: Osi+Chemo vs Osi alone
- mOS 47.5 vs 37.6 mo
- HR 0.77 (95% CI 0.61–0.96; p=0.02)
- 3-yr OS: 63% vs 51%
Benefit consistent across subgroups
- AEs: manageable, Osi discontinuation 12% vs 7%
- Adds ~10 mo survival, but at cost of prolonged pemetrexed and real-world feasibility issues.
Takeaway: Osi+Chemo is new SOC, but Osi mono (mOS 37.6 mo) remains excellent for selected patients”
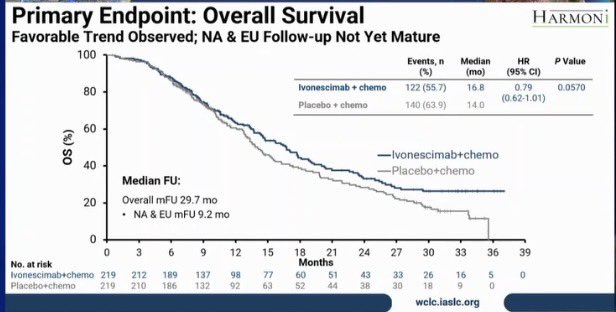
“Data that we all were waiting for (ivonescimab + chemo post-osimertinib):
HARMONi-A at WCLC25 – ivonescimab (PD-1/VEGF bispecific) + chemo vs chemo after 3rd-gen EGFR-TKI progression
- mPFS 6.8 vs 4.4 mo (HR 0.52, p<0.001)
- ORR 44.7% vs 34.2%
- Intracranial PFS benefit (HR 0.34 in BM patients)
- mOS 16.8 vs 14.0 mo (HR 0.79, p=0.057) → trend, not statistically sig.
Grade ≥3 TRAEs: 50% vs 42% (mainly VEGF-related, manageable)
Takeaway: Strong PFS + intracranial signal, OS trending positive but missed significance. Clinically relevant in EGFRm NSCLC post-TKI, esp. for brain mets.
Regulatory and real-world adoption may hinge on further OS update?
Or is this the final full stop?”
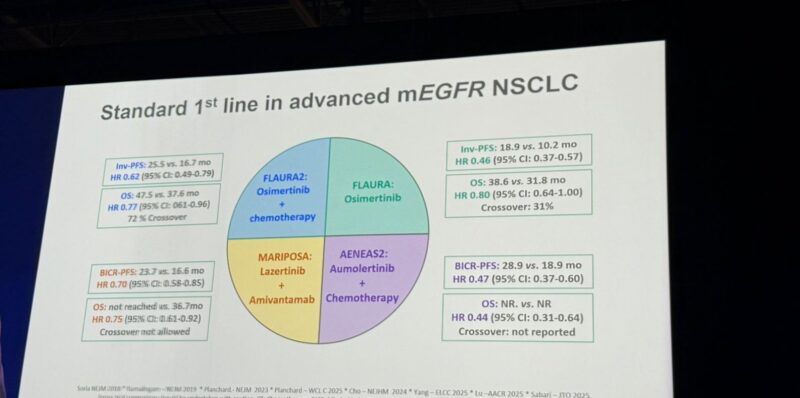
“Excellent slide to show standard 1st line treatment for advanced NSCLC with mEGFR.”
Phase III | 1L EGFRm NSCLC with concomitant tumor suppressor gene (TSG) alterations
Design: Aumolertinib 110 mg QD ± platinum/pemetrexed (induction → maintenance)
N=126 (62 combo / 64 mono)
Median FU: 25.3 mo
Efficacy
- mPFS 19.8 vs 16.5 mo
- HR 0.55 (95% CI 0.34–0.91; p=0.02)
- OS immature
- PFS gain notable in this poor-risk biology (TSG+) group
Safety
- Common AEs ≥20%: cytopenias, ↑AST/ALT, ↑CK, nausea, rash
- No new safety signals, manageable profile
Takeaway: First RCT dedicated to EGFRm + TSG patients Aumolertinib+chemo delivered a statistically significant PFS improvement vs TKI alone, with tolerable safety.
Adds weight to the growing TKI+chemo intensification paradigm (echoes FLAURA2).”
“ARROS-1 at WCLC25 – Zidesamtinib, next-gen ROS1 TKI (brain-penetrant, TRK-sparing)
TKI-pretreated (n=117)
- ORR 44%
- DOR ≥6 mo 84%, ≥12 mo 78%, ≥18 mo 62%
CNS disease: IC-ORR 48%, IC-DOR ≥12 mo 71%
G2032R mutation: ORR 54%, durable responses
- TKI-naïve (n=35, prelim)
- ORR 89% (31/35)
- 12-mo DOR 96%
- IC-ORR 83% (4 CRs), no CNS progression events
Safety (N=432): Mostly mild—edema, constipation, ↑CPK, fatigue. Gr≥3 events rare; 10% dose reduction, 2% discontinuation.
Takeaway: Durable systemic + CNS activity, including in heavily pretreated and G2032R+ patients Zidesamtinib emerging as a strong candidate in resistant ROS1+ NSCLC, with promise even in naïve disease.”
“Is this the end or beginning for ivonescimab?”
Adjuvant crizotinib vs observation in resected stage II–IIIB ALK+ NSCLC
- N=166 (2014–2024, accrual stopped after adjuvant alectinib approval)
- Median FU: 58 mo
- Median DFS: 72.8 mo (criz) vs 75.1 mo (obs)
- HR 1.06 (95% CI 0.63–1.77; p=0.86) → no benefit
- OS: Not reached, HR 0.49 (p=0.26)
Toxicity:
- 34% G3 AEs (diarrhea 6%, edema 4%);
- 1% G4 stroke
- 25% discontinued criz due to AEs;
- median exposure only 13.5 mo
Takeaway: Adjuvant crizotinib failed to improve DFS, with high discontinuation rates. Confirms why 1st-gen TKIs are obsolete in early-stage ALK NSCLC.
Adjuvant alectinib (ALINA) now the true standard.”
More from WCLC25 on OncoDaily.


