Shubham Pant, Professor in the Department of Gastrointestinal Medical Oncology at The University of Texas MD Anderson Cancer Center, shared an article on LinkedIn:
“I’m honored to have had the opportunity to present updated data from the Phase 1 AMPLIFY-201 trial of the ELI-002 KRAS vaccine at the ESMO – European Society for Medical Oncology-IO Conference in Geneva.
Our presentation highlighted longer-term survival data, building on the initial results published in Nature Medicine.
Key findings included:
- 25 patients enrolled (20 PDAC, 5 CRC)
- Patients with KRAS G12D and G12R mutations, no evidence of disease but high relapse risk after resection
- 84% of patients showed biomarker declines (ctDNA, CA 19-9, CEA)
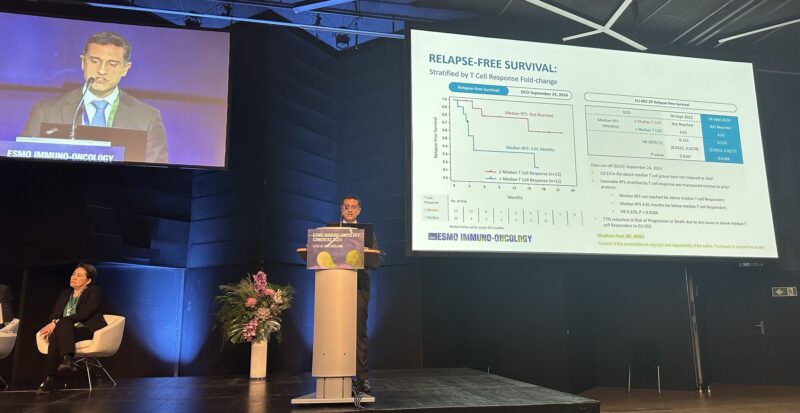
- Median follow-up: 19.7 months | Median RFS: 16.33 months (not reached for patients with ≥ median T-cell response)
Enrollment for the randomized Phase 2 trial is now complete — 135 patients enrolled in just 10 months!
Looking forward to the next phase of Cracking KRAS MD Anderson Cancer Center.
Open Access Article.”
Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial.
Authors: Shubham Pant, et al.

Shubham Pant is a Professor in the Department of Gastrointestinal (GI) Medical Oncology with a joint appointment in the Department of Investigational Cancer Therapeutics (Phase I Center) at The University of Texas MD Anderson Cancer Center. Shubham Pant specialises in the treatment of Gastrointestinal Cancers with an emphasis on Pancreatic and Biliary cancers and Phase 1 trials. His research focuses on novel immunotherapeutic approaches and targeted therapies in GI cancers, including devising novel ways to target the KRAS mutation. He has helped draft the American Society of Clinical Oncology (ASCO) Metastatic Pancreatic Cancer Guidelines. Dr. Pant completed his fellowship from the James Cancer Hospital/Solove Research Institute at the Ohio State University. Dr. Pant has received numerous awards and honors including the Golden Pillar Award for Outstanding Patient Service and the Mai Eager Anderson Endowed Chair in Cancer Clinical Trials.


