Shahrin Ahmed, Medical Specialist at the Canadian Cancer Society, shared a post on X:
“Advanced Synovial Sarcoma has been approved by the FDA as an engineered cell therapy.
Some people with metastatic synovial sarcoma, a type of soft tissue sarcoma, have been given the green light by the FDA to use a cellular therapy called afamitresgene autoleucel (Tecelra). A T-cell receptor (TCR) therapy for cancer has been approved by the agency for the first time in this decision.
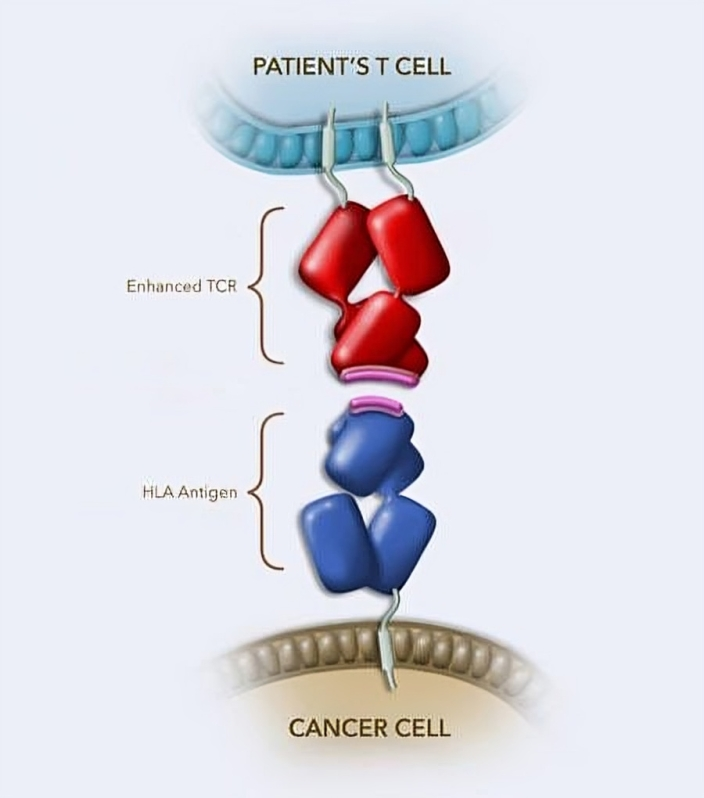
T cells, a type of immune cell, are used to make Afami-cel. The patient’s blood is used to collect and genetically engineer T cells, which are then infused back into them. A T-cell receptor produced through genetic engineering is better at recognizing and binding to MAGE-A4 protein in cancer cells.
44 people with metastatic synovial sarcoma were included in a clinical trial that led to the approval. The treatment caused tumors to shrink in 19 participants (43%), and the median response time, which is when the treatment prevents tumors from growing, was 6 months. Adaptimmune, which is the drug’s manufacturer, sponsored the trial.
Patients with metastatic synovial sarcoma who are eligible for treatment will have access to Afami-cel as their new standard treatment.
Soft tissues like muscles and ligaments are susceptible to synovial sarcoma. It has the potential to occur in extremities and near joints, like the wrist or ankle. The disease is uncommon, with less than 1,000 individuals diagnosed in the United States every year.
Under the age of 30, there are limited treatment options for one-third of people with synovial sarcoma. Treatment is usually surgical to remove the tumor when the disease has not spread within the body. If the tumor is bigger, returns after being removed, or has expanded beyond its original location, radiation therapy and/or chemotherapy may be used.
Metastatic disease is a non-curable condition that affects roughly half of patients with this cancer. Patients with metastatic disease usually have chemotherapy as their standard treatment.
Afami-cel’s production process takes roughly 6 weeks, and it is administered through a patient’s bloodstream in a single dose. The objective of genetic engineering is to address the issue of naturally occurring T-cell receptors not always detecting and binding tightly to cancer cells. The immune cells are able to kill cancerous cells through secure binding.
Nausea, vomiting, fatigue, and infections were the most common side effects of afami-cel. Cytokine release syndrome, a type of immune system overreaction, was experienced by roughly 70% of the participants. According to the researchers, most cases were minor and could be treated with additional medications.”
More posts featuring Shahrin Ahmed.