Sergio Cifuentes Canaval, ASCO International Development and Education Award (IDEA) Recipient – 2025 at Conquer Cancer, the ASCO Foundation, shared a post on LinkedIn:
“Hormone receptor-positive, HER2-negative (HR+/HER2-) breast cancer presents mutations in the PIK3CA gene in approximately 35-40% of cases, which is associated with resistance to endocrine therapies and a worse prognosis in advanced disease. The PI3K pathway, along with estrogen receptors (ER) and CDK4/6 kinases, is key to tumor progression and treatment resistance.
Therapeutic Innovation: Inavolisib
- Inavolisib is a next-generation, highly selective oral inhibitor of the PI3Ka isoform that also promotes the degradation of the mutated p110a protein, improving efficacy and tolerability compared to previous inhibitors.
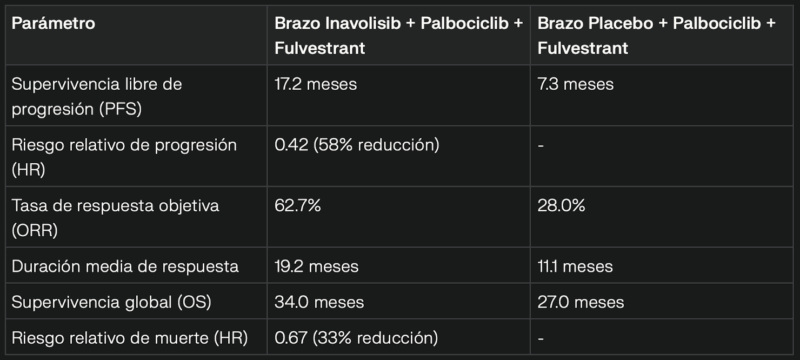
- The INAVO120 study (phase III, double-blind, placebo-controlled) evaluated the combination of inavolisib + palbociclib + fulvestrant in first-line treatment for patients with HR+/HER2- metastatic breast cancer with PIK3CA mutation.
- 325 patients with high tumor burden (80% with visceral metastases, 51.7% with liver metastases) were enrolled.
Key Results
Toxicity and Management
- The addition of inavolisib increased adverse effects such as hyperglycemia (63.4% vs 13.5%), diarrhea (52.2% vs 16%), stomatitis (55.3% vs 28.8%), and ocular toxicities.
- Grade 3-4 hyperglycemia occurred in 6.8% of cases, and grade 3-4 stomatitis occurred in 5.6%. Hyperglycemia was the main reason for dose interruption or reduction.
- Strict glucose monitoring, prophylactic use of metformin in high-risk patients, and multidisciplinary management are recommended.
- The combination was approved by the FDA in October 2024 for patients with HR+/HER2- PIK3CA-mutated breast cancer and endocrine resistance, marking a shift toward personalized first-line therapies.
Limitations and Critical Considerations
- Underrepresented populations: only 0.6% African American patients, with no clear data on Hispanic participation, limiting extrapolation to diverse ethnic groups.
- Patients with diabetes or prediabetes were excluded, leaving uncertainty about safety in these groups, relevant in regions with high metabolic prevalence.
- Low representation of patients with prior treatment with CDK4/6 inhibitors, common in current practice, raises doubts about efficacy in real-life settings.
- Metabolic toxicities require clear management protocols, especially in populations with high obesity and diabetes.
Point of View for Latin America (LATAM)
- The high prevalence of diabetes and obesity in Latin America (up to 30–40% in some populations) poses a challenge for the safe implementation of this therapy, given the high incidence of hyperglycemia associated with inavolisib.
- The limited representation of Hispanic populations in the study makes direct generalization of results difficult, so it is crucial to promote local studies and post-marketing surveillance.
- The infrastructure for advanced molecular testing (NGS and ctDNA), necessary to detect PIK3CA mutations and guide treatment, is still limited in several LATAM countries, which may restrict timely access to this personalized therapy.
- A multidisciplinary approach involving oncologists, endocrinologists, and nutritionists is required to manage metabolic toxicities and optimize outcomes in patients with prevalent comorbidities in the region.
- The approval and use of this therapy in Latin America must be accompanied by education and adverse effect management programs , as well as policies that facilitate access to genomic testing and innovative medicines.
Benefits vs. Risks of Triplet Therapy with Inavolisib
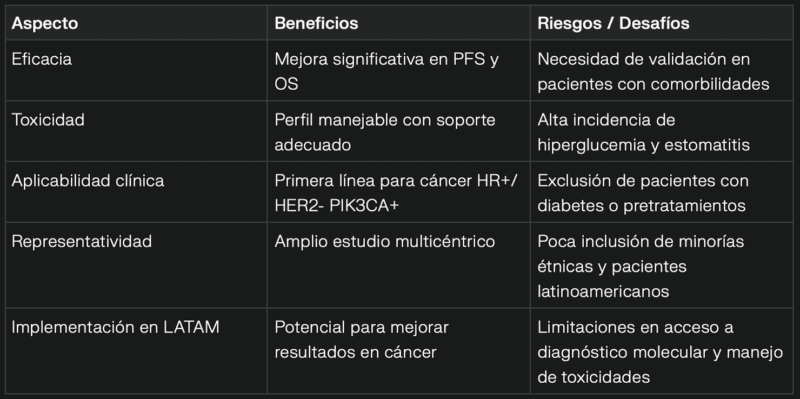
This advance represents a paradigm shift in the treatment of HR+/HER2- metastatic breast cancer, offering a personalized option with clear clinical benefits.
However, its implementation in Latin America must consider the epidemiological, social, and infrastructural specificities to maximize its impact and safety.”
More posts featuring Sergio Cifuentes Canaval.


