Raffaele Colombo, Associate Director of Medicinal Chemistry at Zymeworks, posted on X:
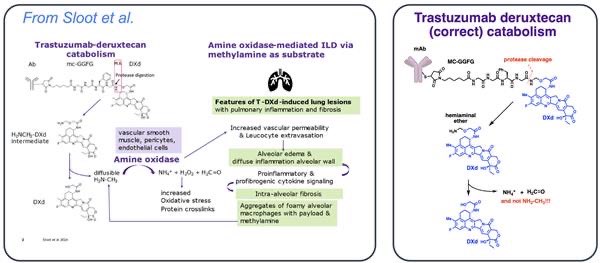
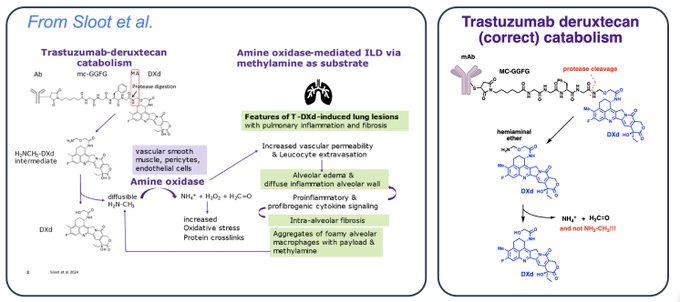
“Sloot et al. published a paper proposing a putative mechanism for ILD, suggesting the involvement of a specific component of the DXd linker (the hemiaminal)
While an understanding of how ADCs can cause ILD is lacking, their explanation has several issues!
Title: The Nonclinical Safety Assessment of a Novel Anti-CEACAM5 Antibody Exatecan Conjugate Predicts a Low Risk for Interstitial Lung Disease (ILD) in Patients—The Putative Mechanism Behind ILD
Authors: Willem Sloot, Elisa Bertotti, Manuela Onidi, Andrea Paoletti, Ilse De Salve, Patrizia Tavano, Enrico Vigna, Gundi Mueller
Read the Full Article.

And the recent publication of M9140 phase 1 data, which amplified this putative ILD mechanism:
Title: Precemtabart tocentecan, an anti-CEACAM5 antibody–drug conjugate, in metastatic colorectal cancer: a phase 1 trial
Authors: Scott Kopetz, Valentina Boni, Ken Kato, Kanwal P. S. Raghav, Maria Vieito, Athanasios Pallis, Christina Habermehl, Abdul Siddiqui, Perrine Courlet, Willem Sloot, Sabine Raab-Westphal, Felix Hart, Ildefonso Rodriguez-Rivera
Read the Full Article.

The authors claim that ADCs with hemiaminal linkers, like T-DXd, yield methylamine (MA) after cleavage by proteases. This is simply wrong. Can’t break chemical bonds like that!

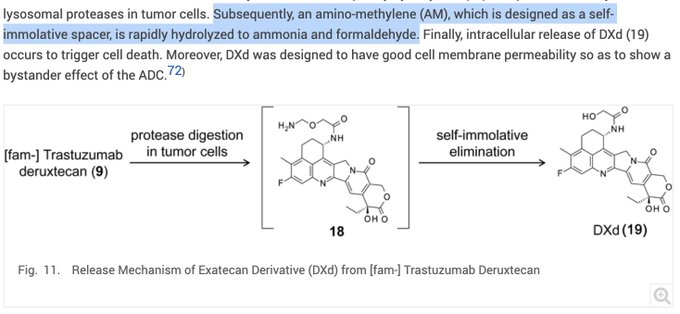
Daiichi reported that for T-DXd metabolism: “an amino-methylene (AM), which is designed as a self-immolative spacer, is rapidly hydrolyzed to ammonia and formaldehyde” Amino-methylene is not methylamine! Read the article.
Sloot et al. further propose the involvement of an enzyme, amine oxidase, to convert methylamine into ammonia, formaldehyde, and hydrogen peroxide.
This is flawed, as methylamine is not formed at any stage in the cleavage pathway of this linker.
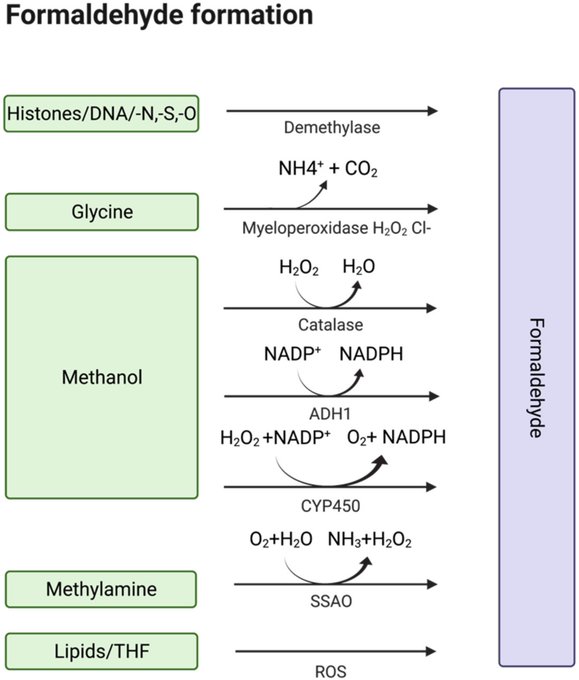
Finally, they speculate that hydrogen peroxide and formaldehyde can cause inflammation and crosslinking. While no hydrogen peroxide is actually formed, formaldehyde is indeed a metabolite and so warrants further discussion.
The formation of formaldehyde as metabolite is possible, but it lacks biological relevance. Our bodies continuously produce formaldehyde through biological processes, and we also intake formaldehyde from food.
Daily endogenous turnover of formaldehyde has been estimated to be ~0.6-0.9 mg/kg per minute (~900-1300 mg/kg per day).
This is even less than the estimated daily intake of formaldehyde from food, which ranges from 1.4 to 1.7 mg/kg body weight per day for a 60-70 kg person.

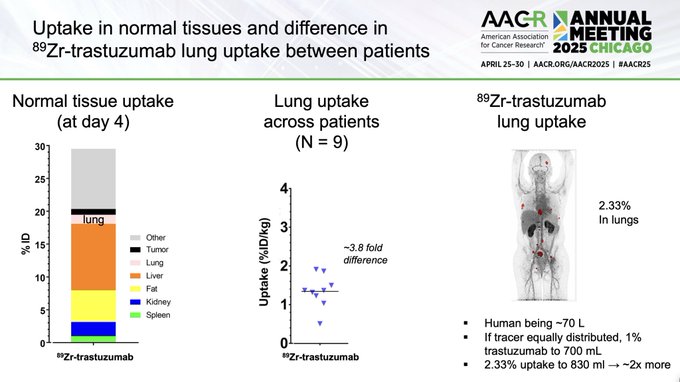
89Zr-trastuzumab lung uptake was low (significant, but still low), meaning the amount of formaldehyde released in the lung is even less than the total 0.0086 mg/kg potentially generated from the ADC.
Slide from Elisabeth de Vries.

For whatever reason, some figures have poor quality. Higher resolution.”

More posts featuring Raffaele Colombo.