Paul Steven, Principal Biostatistician at Precision For Medicine, shared a post on LinkedIn:
“Biomarker Endpoints – the evolution of ctDNA and MRD in Oncology Trials
The future of Oncology trials? Biomarkers leading the charge – faster decisions, smarter designs.
10 years ago, I started with single gene tissue/plasma PCR-based prognostic and predictive tests – used to enrich trials by selecting participants most likely to respond.
Today, with the rise of high-throughput Next-Generation Sequencing (NGS), biomarker use has expanded dramatically. A major advance: detecting Minimal Residual Disease (MRD) via circulating tumour DNA (ctDNA) to predict recurrence and stratify risk.
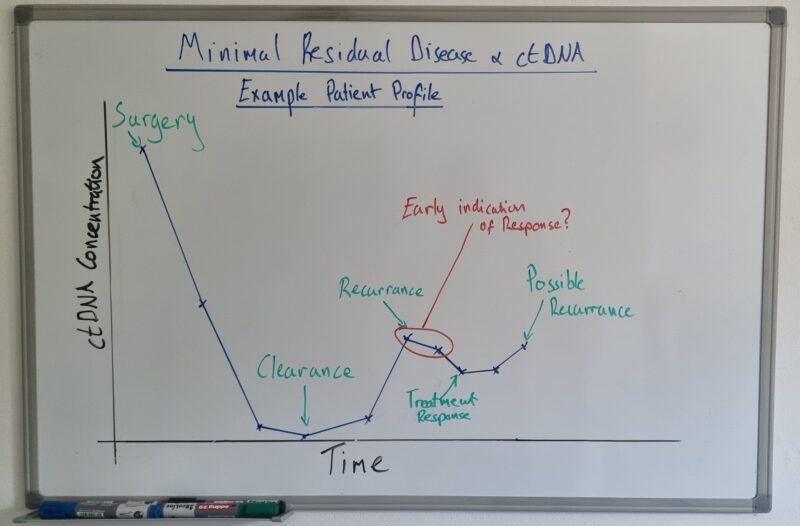
What is MRD?
MRD = small number of cancer cells remaining during/after treatment. Typically detected with NGS-based ctDNA assays (liquid biopsy), but applies to any sensitive method measuring minimal disease burden.
MRD can be diagnostic, prognostic, predictive, pharmacodynamic, or for monitoring. Importantly, there is evidence that MRD can be used as a signal for treatment efficacy before radiological change (RECIST).
Use in Solid Tumors and Early Drug Development
The MRD landscape is more mature in haematological malignancies [1], but it’s rapidly advancing in solid tumours. I’m starting to see ctDNA being positioned as a pre-specified endpoint in early-phase trial SAPs rather than a purely exploratory analysis.
With further development and standardisation, MRD could enable faster, data-driven decisions – imagine adaptively dropping or adding treatment arms based on ctDNA kinetics, rather than waiting to observe radiological change.
Current Use Cases
- Prognostic utility: ctDNA predicts recurrence in multiple tumour types; used in randomisation or as an analysis covariate
- Regulatory signal: FDA has issued guidance for use of ctDNA in curative-intent solid-tumour drug development [2]
Trial Design Considerations
- ctDNA kinetics: Define “clearance” / “increase” / “decrease” using assay precision and %VAF, detection limits
- bAlign with imaging to assess concordance
- Regulatory: If ctDNA guides treatment during trial, consider IUO/IDE implications
Can we define a standardised RECIST-equivalent for ctDNA? Will assay- and tumour-specific calibration be necessary for generalisability?
I would love to hear how you are using MRD and ctDNA in solid tumour trials and beyond.”