FDA shared a post on LinkedIn:
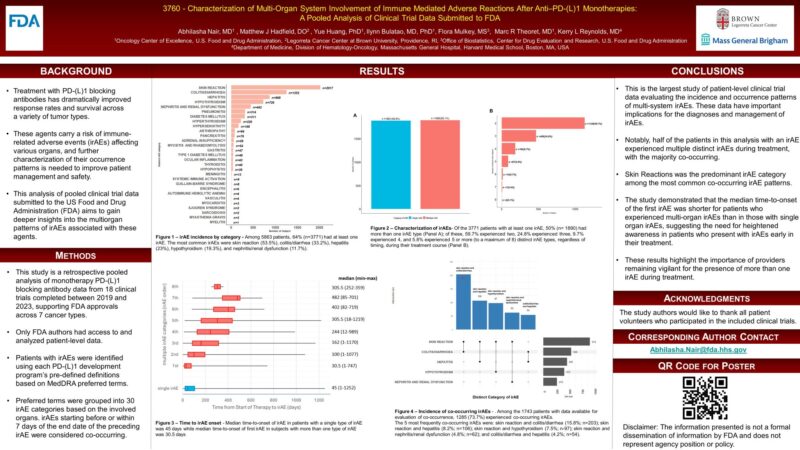
“A descriptive analysis by FDA clinical reviewers using clinical trial data submitted to the agency aimed to uncover insights into the multi-organ patterns of adverse events related to the use of immune system therapies for patients with cancer. The study’s findings may help healthcare providers understand, predict and manage patients experiencing adverse events associated with immunotherapies.
The analysis was published online as part of the American Association for Cancer Research 2025 Annual Meeting.”
Quoting Foundation for Autoimmune Cancer Support (FACS-ASPIRE)‘s post:
“Such important work and amazing to see the FDA dive into immune related adverse events as an overview to have a better understanding the risk profile so we can maximize efficacy of IO.”