Nico Gagelmann, Chair of the CAR-T for plasma cell disorder committee at the EBMT, shared a post on X:
“The EHA 2025 is the prime hematology event in Europe with excellent educational events and top-notch science!m Congrats, European Hematology Association, YoungEHA, for building such an amazing community.
Here are selected abstract presentations to look out for:

Promising new therapy for CALR-mutant ET
Early Phase 1 data show INCA33989, a first-in-class antibody targeting mutCALR, is safe and effective in patients with high-risk ET.
Key Highlights:
- Targets mutCALR in ~25% of ET patients
- 79% overall response rate (66% complete response)
- Platelet count reductions seen as early as 4 weeks
- 88% had decreased mutCALR allele burden
- Well-tolerated with no dose-limiting toxicities or thrombocytopenia
- Most side effects were mild (e.g., fatigue, mild infections)
INCA33989 could represent a precision, mutation-targeted approach for ET patients resistant to current therapies.
A major step forward in MPN care
New results from the Phase III SURPASS-ET trial
Key Highlights:
- 43% of ropeg patients achieved sustained response vs. 6% with anagrelide
- Fewer thrombotic events, disease progression, and serious side effects with ropeg
- Greater symptom control and allele burden reduction
Already approved for polycythemia vera, ropeg now shows promise as a superior second-line therapy for ET.
Promising option for early-stage myelofibrosis
New data show that Ropeginterferon alfa-2b (Ropeg-IFN-α2b) is safe and effective in patients with pre-fibrotic or low/intermediate-1 risk myelofibrosis, a population with limited treatment guidance.
Key Highlights:
- Blood count responses: Hemoglobin – 76.2%
- WBC – 79.4%, Platelets – 100%
- JAK2V617F VAF reduction: 44%
- CALR VAF reduction: 43%
- Spleen size reduction: 53%
- Symptom improvement (≥50% MPNSAF-TSS reduction): 42%
- Most side effects were mild to moderate, including fatigue, hair loss, and mild liver enzyme elevations
Ropeg-IFN-α2b shows strong clinical, hematologic, and molecular activity in early-stage MF, with a favorable safety profile and potential to delay disease progression.
Big news in multiple myeloma
Early results from the Phase 1 study of JNJ-5322, a next-gen trispecific T-cell redirecting antibody, show remarkable efficacy in RRMM.
Key Highlights:
- JNJ-5322 targets BCMA + GPRC5D with a low-affinity CD3 domain to maximize tumor attack while minimizing side effects
- At the recommended dose (100 mg Q4W):
- 100% response rate in patients naïve to BCMA/GPRC5D therapies
- 86% overall response rate in treated patients
- Most CRS events were mild (no grade ≥3)
- Lower GPRC5D-related side effects vs other bispecifics
A potential CAR-T-like response, without the complexity of cell therapy.
Congrats, Rakesh Popat et al.
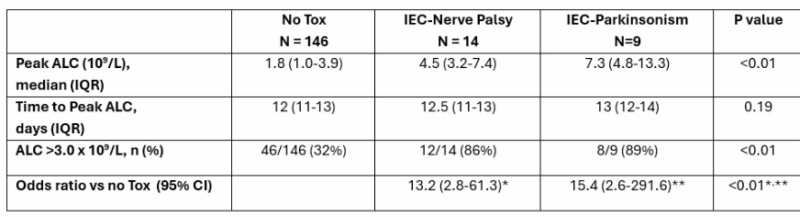
Cilta-cel and Delayed Neurotoxicity: Key Risk Factor Identified
Key Highlights:
- 235 patients, 9.7% developed delayed neurotoxicity
- 3.8% IEC-PKS, 6.4% IEC-NP
Top predictor:
- Peak ALC ≥3 x10⁹/L post-infusion → 30% risk vs 3% without
Other risks for IEC-PKS:
- Age >75, high tumor burden, ICANS, elevated ferritin
Treatment:
- IEC-NP: 85% responded to steroids
- IEC-PKS: Early cyclophosphamide led to rapid symptom relief
Peak ALC may guide early intervention to prevent or manage cilta-cel neurotoxicity. Congrats Rafael Fonseca, Yi Lin et al.
Breakthrough for extramedullary disease in myeloma
New Phase 2 data from RedirecTT-1 show that combining Talquetamab + Teclistamab delivers strong, durable responses in RRMM patients with extramedullary disease – a group historically resistant to treatment.
Key Highlights:
- 90 patients
- 79% overall response rate (52% ≥ complete response)
- 83% ORR in patients with prior CAR-T therapy
- 9-month PFS: 64% | OS: 80%
- Most responses deepened/held after switching to monthly dosing
- CRS in 78% (all grade 1/2)
- ICANS in 12% (mostly mild)
- Common AEs: taste changes, skin/nail effects, infections (mostly early and manageable)
This is the largest EMD-specific study to date, and results rival those of CAR-T, with off-the-shelf accessibility.
New Approach for High-Risk MDS: BEXMAB Trial Results
Bexmarilimab, a first-in-class macrophage checkpoint inhibitor, shows promising early results in combination with azacitidine for patients with high-risk myelodysplastic syndrome (HR MDS)—a group with few effective options, especially after HMA failure.
Key Highlights:
- 100% response rate in initial frontline MDS patients
- 80% ORR in relapsed/refractory (r/r) MDS (65% by updated IWG2023 criteria)
- Median OS of 13.4 months in r/r MDS—more than double historical outcomes (<6 months)
- No dose-limiting toxicities in dose escalation
- Most AEs were manageable; only 13.7% were related to bexmarilimab
- Doses of 3mg/kg and 6mg/kg being evaluated for optimal efficacy/tolerability
The BEXMAB study offers a potential immunotherapy-based breakthrough for both frontline and r/r HR MDS, patients with urgent unmet need.
Congrats, Amer Zeidan, Naval Daver et al.
New hope for BPDCN patients
The Phase 1/2 CADENZA trial shows that PVEK, a first-in-class CD123-targeting antibody-drug conjugate, delivers high response rates and manageable safety in blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Key Highlights:
- 70% CR + CRc rate
- 85% overall response rate
- Median OS: 16.6 months
- 92% response rate in those bridged to stem cell transplant
Relapsed/Refractory (R/R) Results:
- 14% CR + CRc | 35% ORR
- Responses similar to or without prior tagraxofusp
Safety Snapshot:
- No capillary leak syndrome or treatment-related deaths
- Most common side effect: low-grade peripheral edema
- Rare, reversible veno-occlusive disease (2%)
Given as a short IV outpatient infusion every 21 days, PVEK could be a powerful new option for both newly diagnosed and previously treated BPDCN patients.
Congrats, Naveen Pemmaraju.”
More posts featuring Nico Gagelmann.


