Piotr Wysocki recently shared on LinkedIn:
“Zhou Y et al. have conducted an indirect meta-analysis evaluating the role of adjuvant immunotherapy in patients with resectable NSCLC who underwent neoadjuvant antiPD1/PDL1-based treatment.
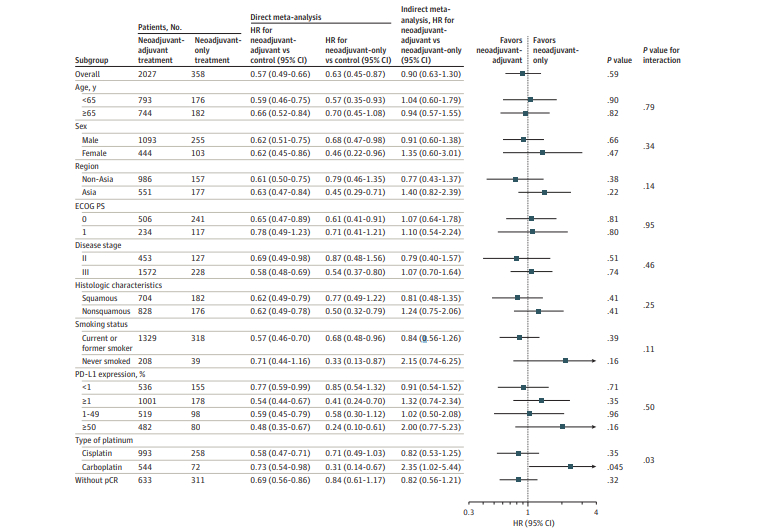
The analysis included 2385 patients from 5 clinical trials. Four trials (KEYNOTE-671, Neotorch, AEGEAN, and NADIM II) explored neoadjuvant-adjuvant immunotherapy vs chemotherapy alone, while CheckMate 816 investigated neoadjuvant-only immunotherapy. All included trials involved patients with a diagnosis of stage II to III resectable tumors except for NADIM II, which focused exclusively on patients with stage III tumors.
Results
Direct meta-analysis
- Neoadjuvant-adjuvant immunotherapy was significantly better than chemotherapy alone (HR for EFS= 0.57; 95% CI 0.49-.66)
Indirect meta-analysis
- Neoadjuvant immunotherapy followed by adjuvant immunotherapy is not better than neoadjuvant-only immunotherapy (HR for EFS=0.90; 95%CI 0.63-1.30; P = .59)
- Patients not achieving pCR may derived slight, albeit nonsignificant, improvement in EFS with additional adjuvant therapy (HR=0.82; 95%CI 0.56-1.21; P = .32)
- Patients receiving a carboplatin-based chemotherapy regimen have a significantly inferior EFS outcome with adjuvant immunotherapy (HR=2.35; 95%CI 1.02-5.44; P = .045).
- Both neoadjuvant-adjuvant and neoadjuvant-only immunotherapies significantly improved OS compared with chemotherapy alone (HR for OS = 0.67; 95%CI 0.52-0.85).
- Comparing neoadjuvant-adjuvant and neoadjuvant immunotherapies, the addition of adjuvant immunotherapy significantly increased the incidence of treatment-related adverse events (TRAE) of any grade (RR=1.08; 95% CI, 1.00- 1.17; P = .04).
- Fatal TRAEs reported in trials varied between 1.0% and 3.0% in the neoadjuvant-adjuvant immunotherapy group, whereas no fatal TRAEs were reported in the neoadjuvant-only immunotherapy groups.
The Zhou Y et al. meta-analysis provides important information indicating that neoadjuvant immunotherapy-based treatment represents a crucial part of perioperative systemic treatment in NSCLC patients. A modest benefit of adjuvant immunotherapy may be only expected in patients with residual disease, albeit increased toxicity associated with adjuvant treatment may preclude the achievement of long-term benefits.”
Authors: Yixin Zhou, Anlin Li, Hui Yu, MD; Yuhong Wang, MD; Xuanye Zhang, MD; Huijuan Qiu, MD; Wei Du, MD; Linfeng Luo, Sha Fu, Li Zhang, Shaodong Hong,
Source: Piotr Wysocki/LinkedIn
Piotr Wysocki leads the Clinical Oncology Department at University Hospital and the Faculty of Oncology at Jagiellonian University-Medical College in Krakow, Poland. As an advisor to the Polish Ministry of Health, he shapes the national cancer strategy.
His clinical expertise spans the systemic treatment of breast, gynecologic, and genitourinary cancers, with a focus on developing innovative metronomic chemotherapy-based therapies for advanced cancer patients who have undergone prior treatment.
Read other posts by Piotr Wysocki published on OncoDaily.