Toni Choueiri shared on X/Twitter:
“The long-awaited OS results from KEYNOTE-564 are out at the New England Journal of Medicine! The 1st phase 3 trial to demonstrate a survival benefit for adjuvant treatment in RCC.
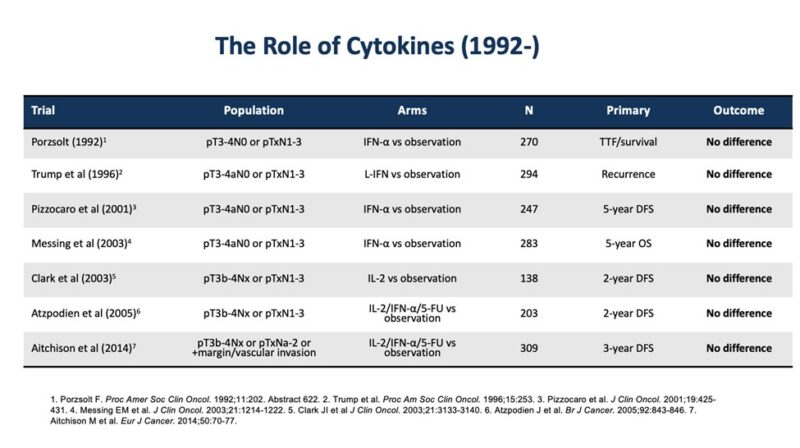
After 50 years of adjuvant trials on RCC (3 slides); KEYNOTE564 demonstrates overall survival (OS) benefit!
KEYNOTE-564 is a phase 3, double-blind, randomized, placebo-controlled trial.
– ccRCC
– ECOG PS 0-1
– ≤12 weeks following surgery
– Intermediate-high, high risk M0, or M1 NED (within 1 year of nephrectomy)
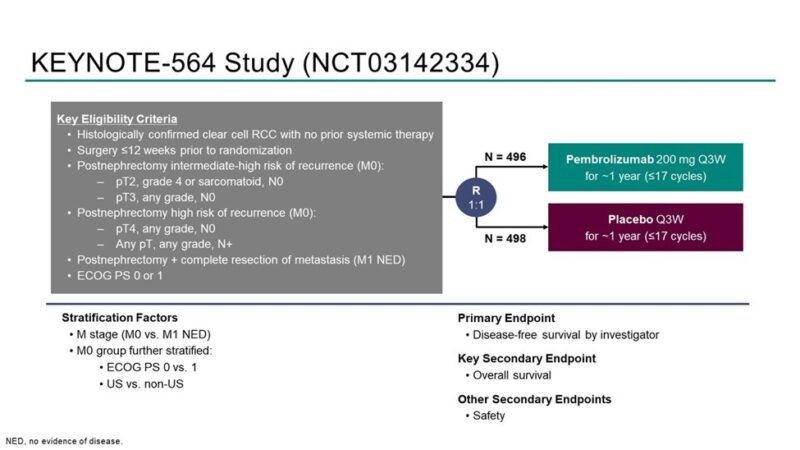
Patiens were randomized 1:1 to receive pembrolizumab 200mg Q3W for 1 year or placebo.
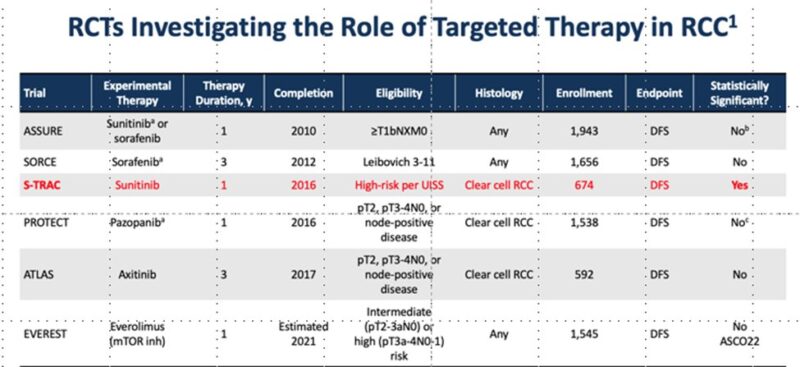
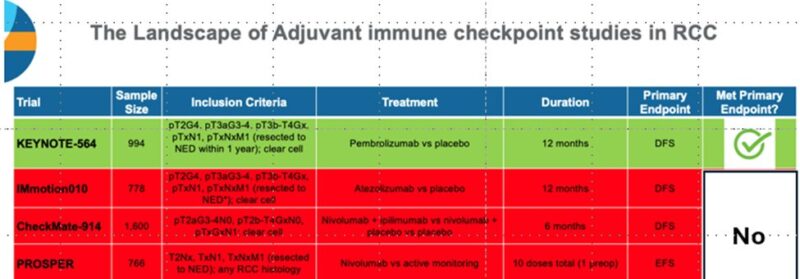
Primary endpoint: DFS (investigator) – met at 1st interim analysis, published in New England Journal of Medicine 2021 and Lancet Oncology 2022 by partner Tom Powles.
Key 2nd endpoint: OS : here!
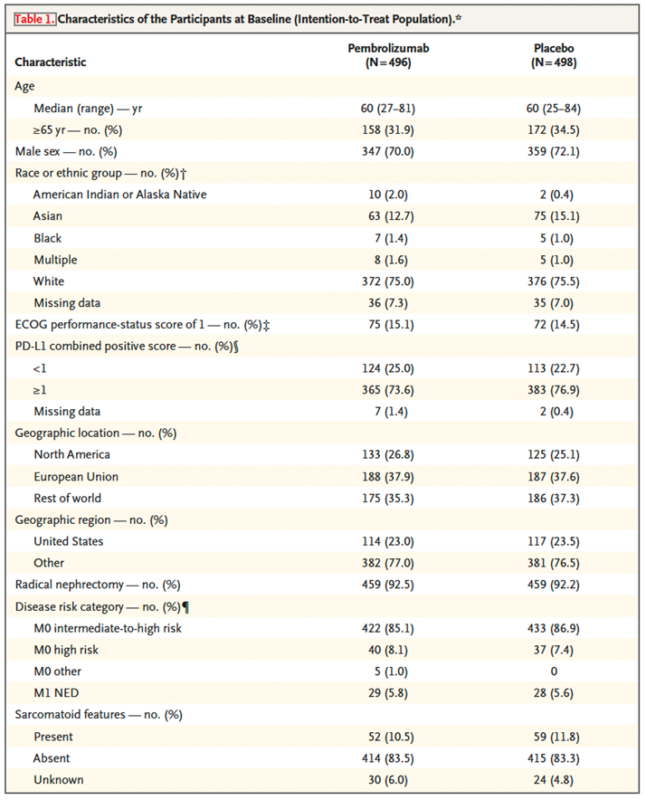
994 patients enrolled: 496 pembrolizumab vs 498 placebo. Median time from rand. to data-cutoff (Sep 15 2023): 57.2 months (range 47.9-74.5, close to 5 years)
Baseline characteristics:
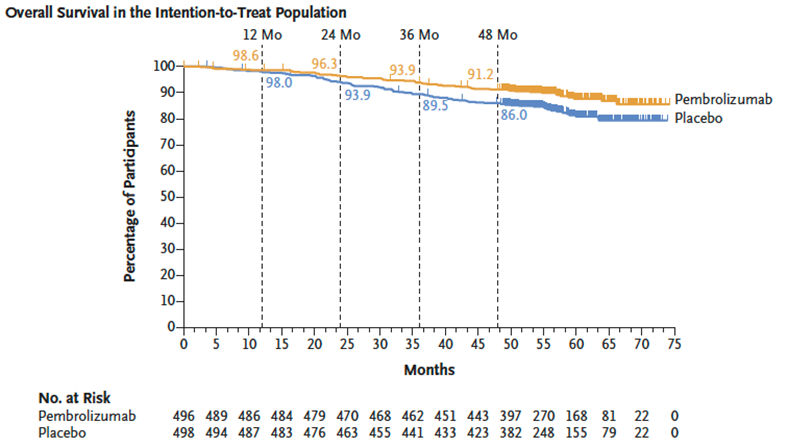
A significant and clinically meaningful OS benefit was observed with adjuvant pembro vs placebo:
HR 0.62; 95% CI 0.44-0.87; P = 0.005
At 48 months: 91% of patients receiving pembrolizumab were still alive vs 86% with placebo (5% absolute benefit).
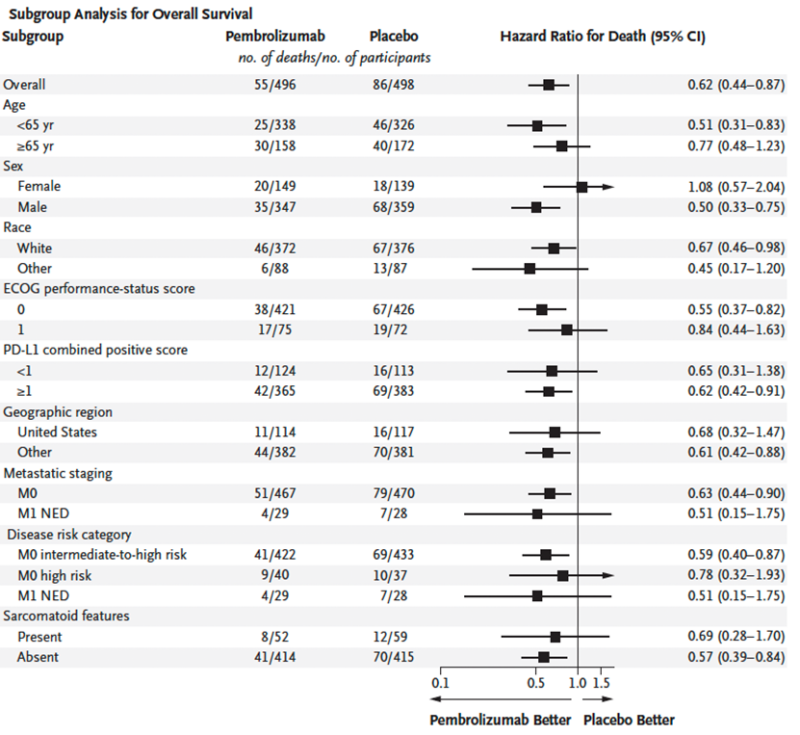
OS benefit was consistent across subgroups.
Of note, the OS benefit was consistent in the M0 intermediate-high risk patients (HR 0.59; 95% CI 0.40 to 0.87) while sample sizes and number of deaths were small in the M0 high risk patients and M1 NED, leading to wider CIs.
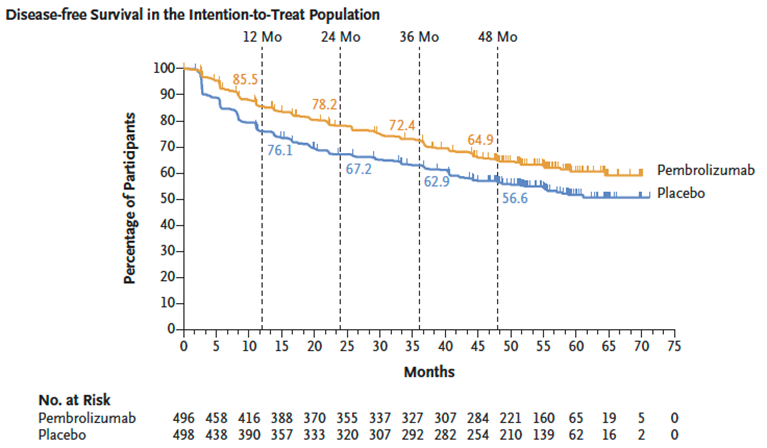
Updated DFS results aligned with previous analyses:
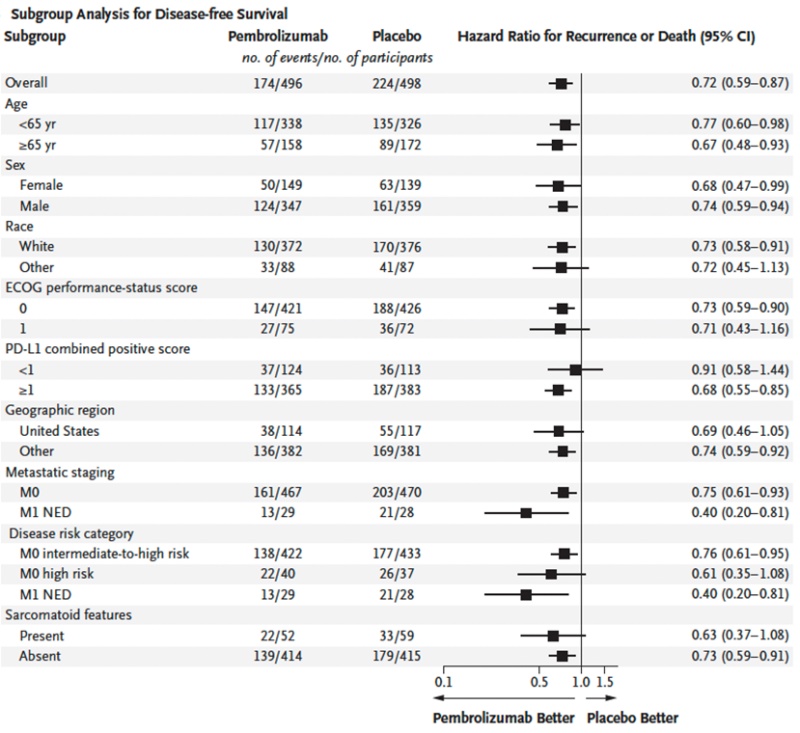
DFS benefit for pembrolizumab: HR 0.72 (95% CI 0.59 to 0.87), generally consistent across subgroups.
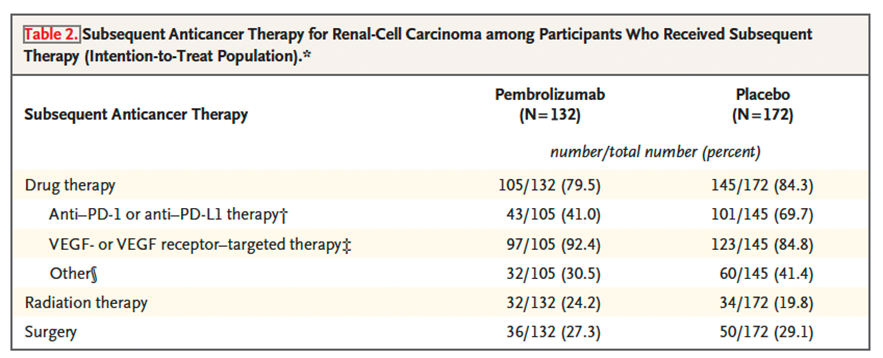
The rates of patients receiving subsequent therapies, consisting in radiation, surgery or systemic treatment, are displayed below.
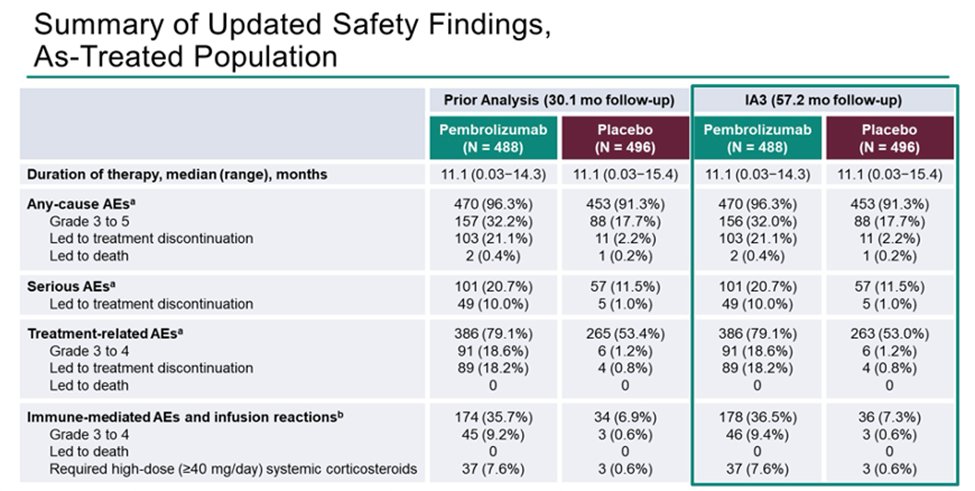
Safety findings were consistent with prior analyses (as all patients had d/c therapy since Dec 2020):
Huge thanks to all the investigators, my partner Tom Powles, to Merck, and mostly to our patients/families, to whom we dedicate all our efforts!
Balanced editorial by Martin Voss and Robert Motzer.”
Source: Toni Choueiri/X
Toni Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director of International Strategic Initiatives at Dana-Farber and past President of the Medical Staff at DFCI (2016-2018).
He received the George Canellos Award for Excellence in Clinical Investigation and Patient Care from DFCI in 2013, the Eugene Schonfeld Award from the Kidney Cancer Association (KCA) in 2016, and is a 2021 Giants of Cancer Care inductee. He serves on the National Comprehensive Cancer Network (NCCN) Kidney Cancer Panel, KidneyCan Board, the National Cancer Institute (NCI) GU Steering Committee, and is past Chairman (2015-2018) of the Medical and Scientific Steering Committee of the KCA.
Dr. Choueiri is an elected member of the American Society of Clinical Investigation (ASCI). In addition, he is an Aresty Scholar from the Wharton School of Business at the University of Pennsylvania.
About OncoDaily
OncoDaily was founded in 2023. It is a US-based oncology media platform, which features the latest news, insights, and patient stories from the world of oncology. Within a short period of time it became one of the leading oncology media platforms globally.
OncoDaily gathers content from various sources, including social media posts from renowned oncologists from all over the world, news from oncology societies and cancer centers, patient and survivor stories, and career-related information for professionals.
The mission of OncoDaily is to empower patients, survivors, and professionals with the knowledge and inspiration they need to fight cancer. The motto of OncoDaily is “Cancer doesn’t take a day off – neither do we”.