Annals of Oncology shared a post on X:
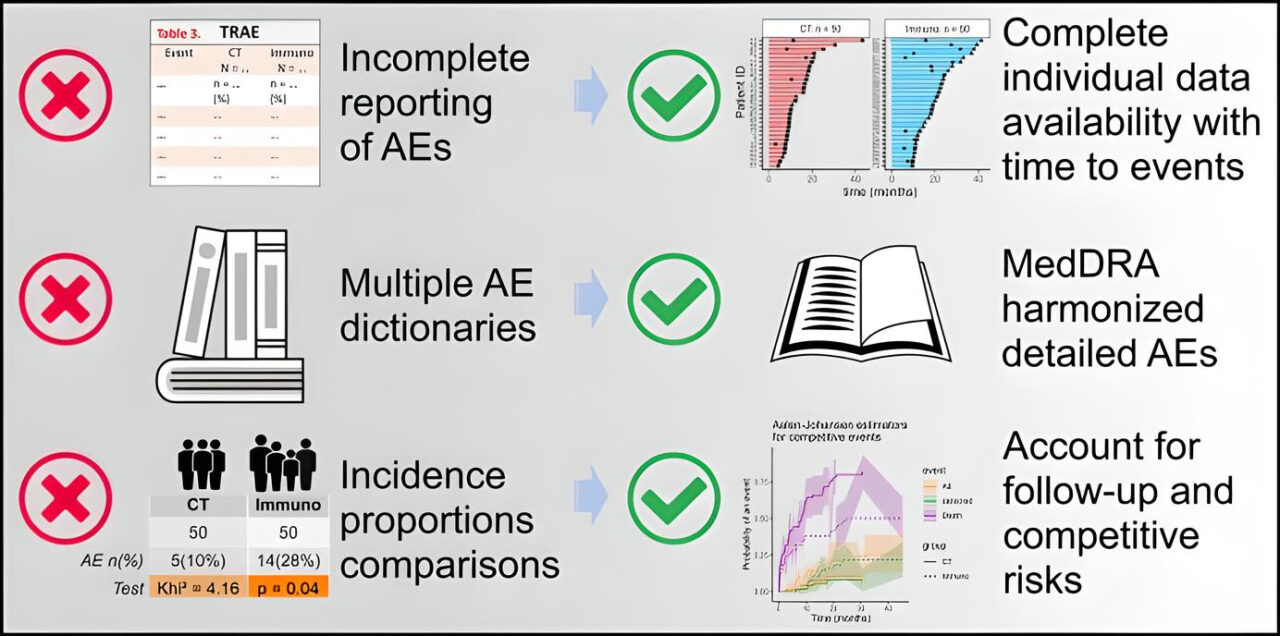
“Immune checkpoint inhibitors are now standard-of -care in a large number of tumor types, as single-agents or part of combinations. The transition from a clinical trial to a ‘real-world’ setting brings challenges regarding the management of adverse events and their reporting.
In this Letter to the Editor, the Cancer Immunotherapy AEs Working Group details the pitfalls of reporting in immunotherapy clinical trials and proposes solutions to address these issues now that these agents are also used in the early setting.”
Read further.
Source: Annals of Oncology/X