Amol Akhade, Consultant medical oncologist at Suyog Cancer Clinics, shared on X/Twitter:
“What is the approach of Indian Oncologist Towards Drug approval agencies? India has its own drug approval agency (unlike many other LMICs). But dose it’s opinion matter to Indian oncologist?
Probably NO.
We conducted an online survey with two simple questions to understand this.
224 oncologist participated in this online survey.
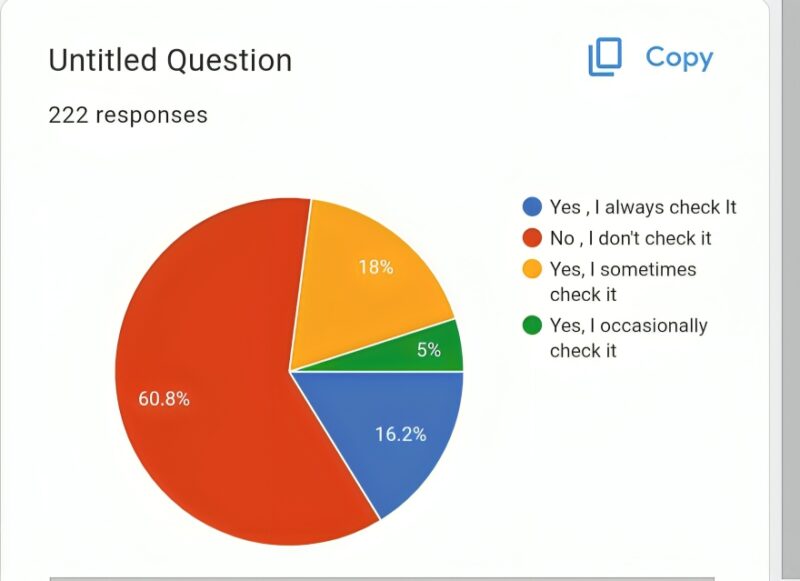
60.8 % oncologist do not even check if drug is approved by Indian Agencies, if It is already approved by US FDA and EU Medicines Agency.
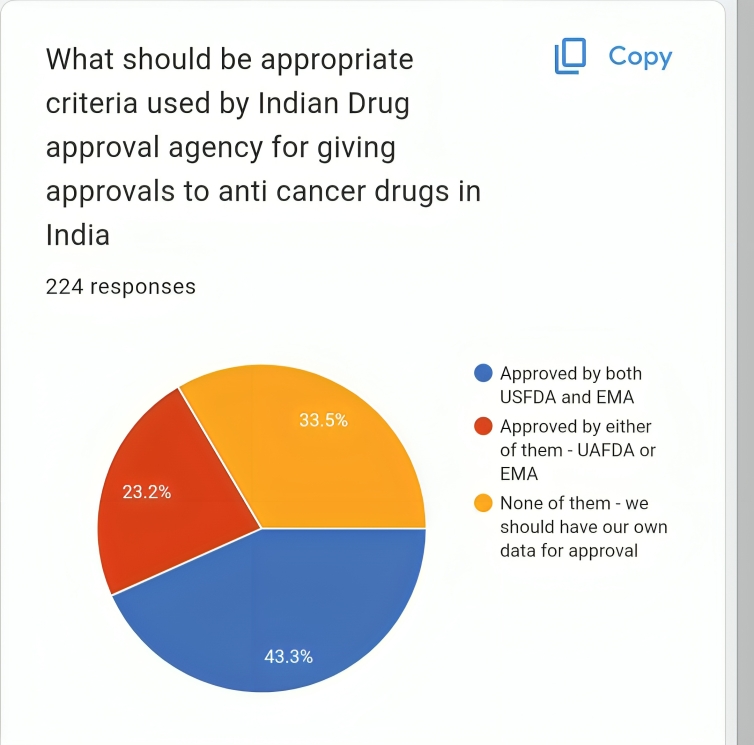
43.3 % prefer if it is approved by both of them (US FDA and European Medicines Agency)
Only 33.5 % feel that we should generate our own data before we give such approvals In India.
Such online surveys have limitations (social media bias – those oncologist who are more active on social media are more likely to participate and the sample size of 224 is relatively small).
Inspite of this,
Does this probably reflects more apathy and lack of trust of Indian Oncologist Towards own drug approval agency?
What can be the reasons?

Source: Amol Akhade/X


