Myriam Chalabi, Medical Oncologist and Cancer Immunotherapy researcher at Antoni van Leeuwenhoek, The Netherlands Cancer Institute, shared a post by Nature Medicine on X, adding:
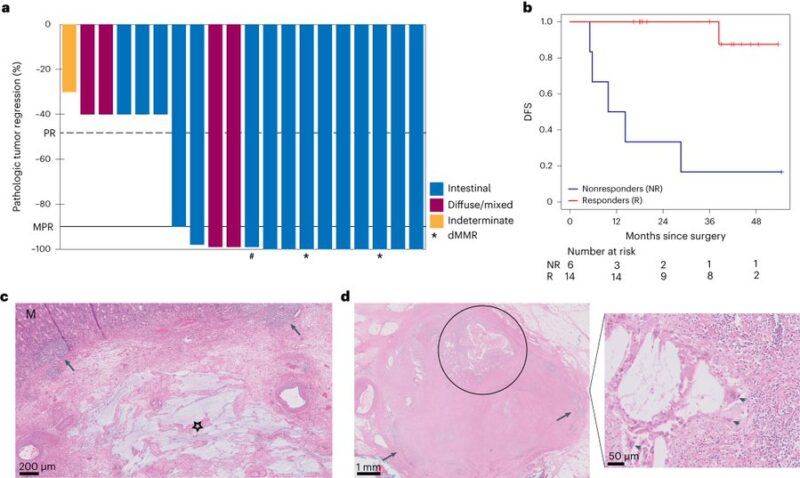
“Check out our paper in Nature Medicine! Highlighted during GI24 Neoadj 1x atezo —> 4x atezo+CTx. No unexpected toxicities for CTx and very limited added IO tox. 70% MPR, 45% pCR. 13/14 responders alive and NED, 5/6 nonresponders had died.
Treatment was neoadjuvant and not peri-operative. All patients underwent all pre-op CTx cycles: means more cumulative neoadjuvant chemo doses in PANDA (exc docetaxel=). While post-operative SoC chemotherapy not completed in >50% of patients.
Why capecitabine? Convenient, usually less toxic, patient-friendly, and at least as effective (possibly somewhat superior?) compared to 5FU.
SoC is 16 weeks (completed in 50% of pts) of FLOT/8x 2-weekly cycles peri-op. But, if we achieve 70% MPR/45% pCR with PANDA chemo doses (DOC), we owe it to pts to consider less chemo if equally/more effective when all neoadjuvant + anti-PDL1.
Also, check out our tweetorial for the translational work!
Working on sequel so PANDA to validate data. Stay tuned.”
Quoting Nature Medicine‘s post:
“A neoadjuvant treatment regimen of anti-PD-L1 immunotherapy followed by anti-PD-L1 + chemotherapy was well tolerated and led to pathologic response rate of 70% in patients with resectable Gastric Cancer or GEJ adenocarcinoma”