
Pashtoon Kasi Highlighted Study on Vilastobart (XTX101) + Atezolizumab in MSS Colorectal Cancer at ASCO 2025
Pashtoon Kasi, Medical Director of GI Medical Oncology at City of Hope Orange County, shared on LinkedIn:
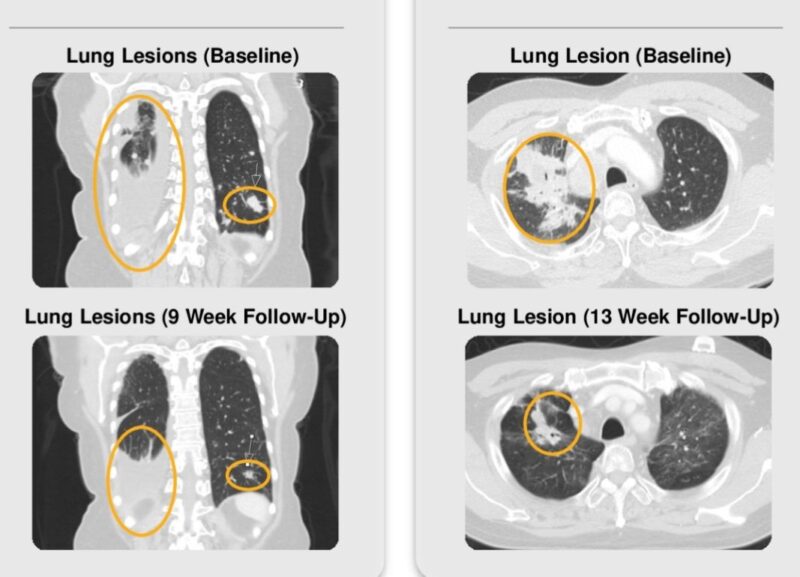
“ASCO 2025 at some of these responses! These are patients with MSS (“cold tumors”), not the MSI-High (“hot tumors”). This is a big unmet need in colorectal cancer and other cancers.
Vilastobart (XTX101)
Tumor-activated CTLA4
PDL1 –Work led by Dr. Marwan Fakih.”
Title: Vilastobart (XTX101), a tumor-activated, Fc-enhanced anti–CTLA-4 monoclonal antibody, in combination with atezolizumab in patients with MSS CRC
Journal: ASCO, Journal of Clinical Oncology
Authors: Marwan Fakih, Nicholas DeVito, Aparna Parikh, Tanios Bekaii-Saab, Hao Xie, Sandra Algaze, Alexander Spira, Jeremy Jones, Cesar Perez, Andrae Vandross, Alberto Bessudo, John Knecht, Ekta Patel, Damiano Fantini, Scott McConnell, Myra Popejoy, Bruce Dezube, Diwakar Davar, Joel Hecht
Timothy Allen, Chief Scientific Officer at the Nexus Alliance Biopharmaceuticals, shared a post by Pashtoon Kasi on LinkedIn, adding:
“Emerging Immunotherapy Opportunity in MSS Colorectal Cancer Without Liver Metastases
Microsatellite stable (MSS) colorectal cancer represents ~85% of all CRC cases and is typically non-responsive to immunotherapy due to low tumor mutational burden and immune exclusion. However, preliminary data now suggest a biologically actionable subgroup—patients with MSS CRC without liver metastases—may derive meaningful benefit from immune checkpoint inhibitors.
In a recent cohort, the objective response rate (ORR) reached 26% in patients without liver metastases, while 0% ORR was observed in those with hepatic involvement. Confirmed partial responses, including >70% tumor reduction, were seen across KRAS-mutant and wild-type populations. Radiologic data (CT) confirmed substantial shrinkage of abdominal and pulmonary lesions within 9–18 weeks.
These findings align with established evidence that liver metastases suppress systemic anti-tumor immunity through tolerogenic antigen presentation and T-cell sequestration (Knolle et al., Nat Rev Immunol, 2014; Yu et al., Front Immunol, 2021). Prior work across melanoma and NSCLC similarly shows reduced efficacy of ICIs in patients with hepatic spread (Tumeh et al., Cancer Discov, 2021).
Current treatment options for MSS mCRC remain limited to chemotherapy (FOLFOX/FOLFIRI ± bevacizumab) and anti-EGFR agents (RAS WT only). ICIs are approved only in MSI-H CRC based on KEYNOTE-177 and CheckMate-142. Trials of bispecific antibodies (CEA-CD3), TGF-β inhibitors, and TLR agonists are ongoing.
From an HEOR perspective, treating all MSS CRC patients with immunotherapy is not cost-effective (ICER >$200,000/QALY). However, response-constrained subgroups like this may justify tailored reimbursement and focused trial design, especially if survival benefits are confirmed (Carlson et al., Health Aff, 2018).
This data supports a redefinition of “cold tumors” and opens the door for stratified MSS CRC immunotherapy trials excluding liver metastases. Precision targeting may finally translate to durable benefit in this historically unresponsive population.”
More posts featuring Pashtoon Kasi on OncoDaily.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
