Talha Badar, Assistant Professor of Oncology at Mayo Clinic Comprehensive Cancer Center, posted on X/Twitter:
“Weekend review:
Brief review of menin inhibitors in clinical trials.
KMT2Ar AL characterized by aberrant overexpression of HOX genes
menin is necessary for KMT2A to bind HOX gene for leukemogenesis
Similarly with NPM1 and NUP98-rearranged.
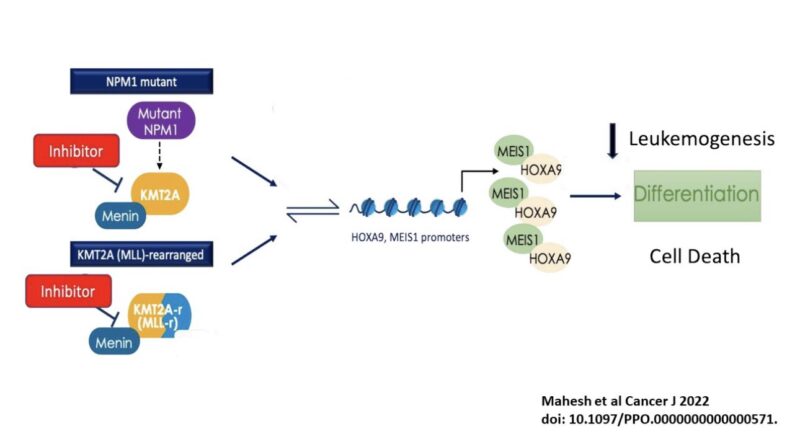
How menin inhibitors work?
Inhibits menin-KMT2A protein-protein interaction induce differentiation
At least 5 different menin inhibitors are being developed: including SNDX-5613 (Revumenib), KO-539 (Zeftomenib), JNJ-75276617, BMF-219, DS-1594a
Revumenib: phase 1 study, 2 arms with and without CYP3A4 inhibitor.
68 pts treated; 82% AML, 16% ALL
68% KMT2A, 21% NPM1
DLT was QTc prolongation.
CR/CRh 30%, median DOR 9.1 mo
67% proceeded to allo-HCT.
AUGMENT 101 phase 2 dose expansion in pediatric and adult R/R KMT2Ar acute leukemia ASH23
57 evaluable patients, ORR 63%, CR/CRh 23%, CRc 44%,
AEs: DS 28%, QTc prolongation 26%, did not let to study discontinuation.
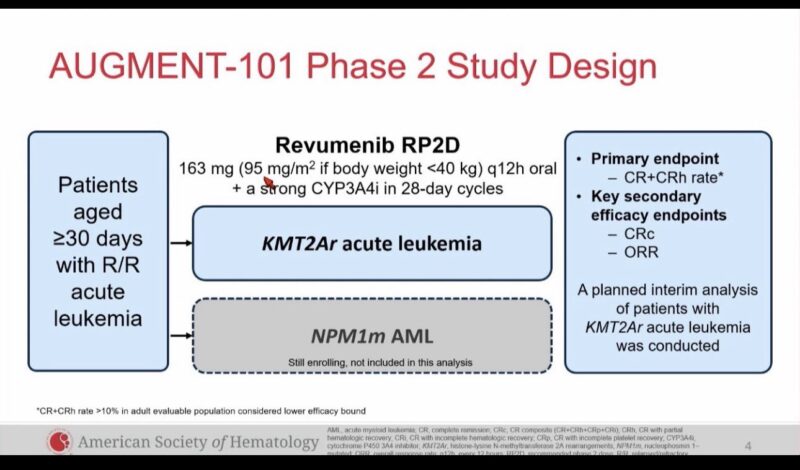
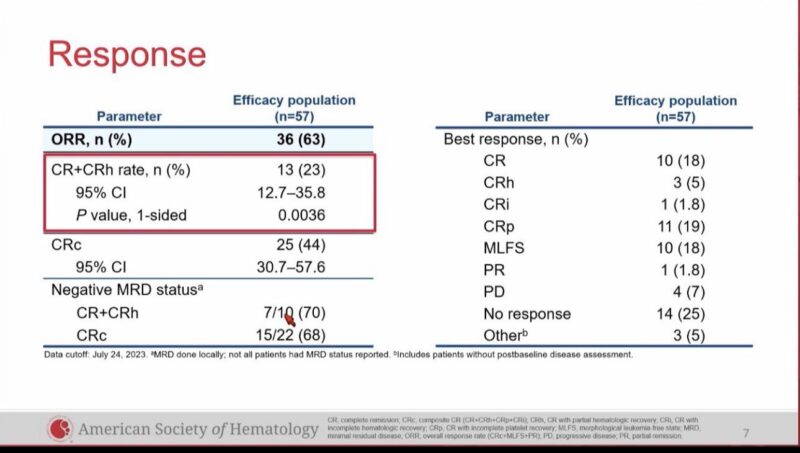
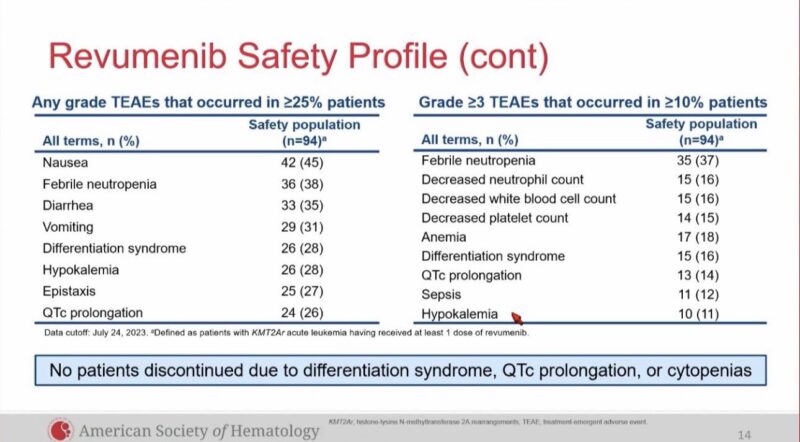
SAVE trial of Revumenib with HMA plus venetoclax
7/8 evaluable pts ORR 100%, all pts achieved morphological remission. 3 bridge to allo-HCT.
2 patient died, one from allo-HCT complications.
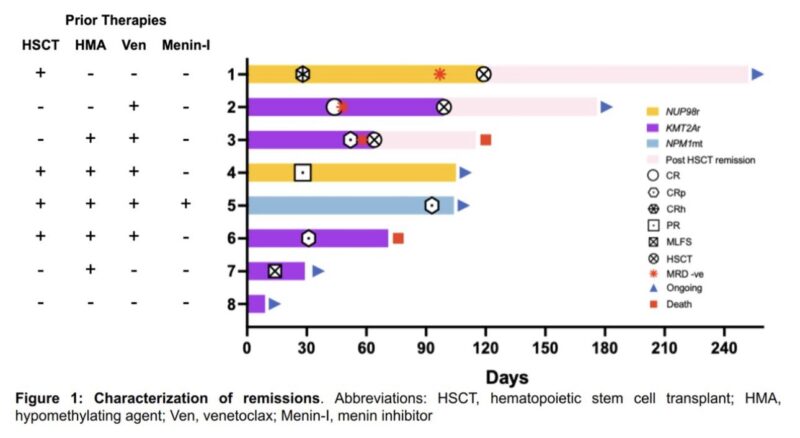
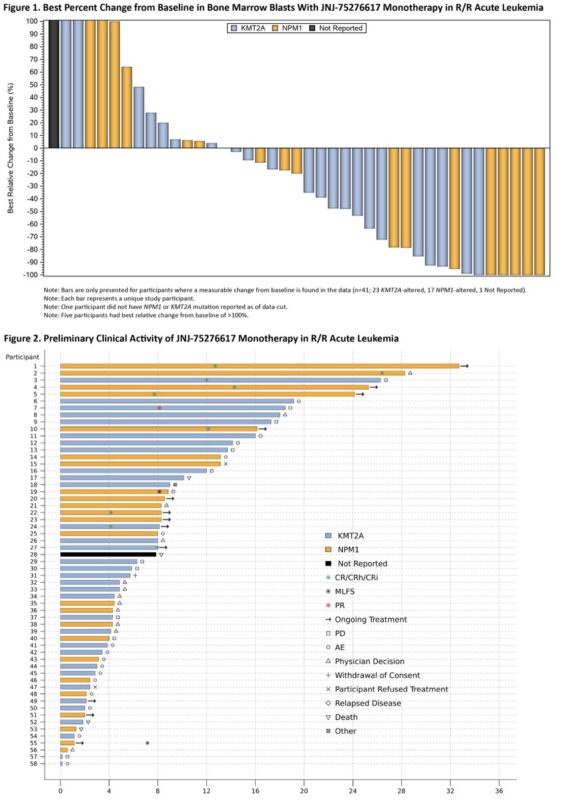
First-in-Human Phase 1 Study of the Menin-KMT2Ai JNJ-75276617 in Pts with RR Acute Leukemia with KMT2A or NPM1 Alterations
58 pts treated, DLT was differentiation syndrome, 1 pt each had CR and CRh. 2 pts had CRi
Dose expansion phase ongoing
KOMET-001 trial (NCT04067336) of Ziftomenib in RR AML with NPMm
CR 30% at 600 mg, mainly in NPM1m AML pts. Low level of differentiation syndrome.
Phase 2 dose expansion ongoing.
Single agent activity in KMT2Am not observed, studies ongoing to evaluate drug in combination
Covalent Menin Inhibitor in Pts with RR AL Preliminary Phase 1 Data
26 pts with R/R AL (24 AML; 2 ALL) are enrolled: no DLTs.
Responses is seen with strong CYP3A4i, in NPM1m and NUP98-rearranged AML.
Dose expansion phase ongoing in combination with strong CYP3A4i
In conclusion
While there is excitement around menin inhibitors, single agent activity is modest.
QTc prolongation and DS are common DLTs
Better responses seen in NPM1m and with strong CYP3A4i
Upfront combination therapy worth exploring, while keeping eye on toxicity.”
Source: Talha Badar/X


