Vivek Subbiah shared on LinkedIn:
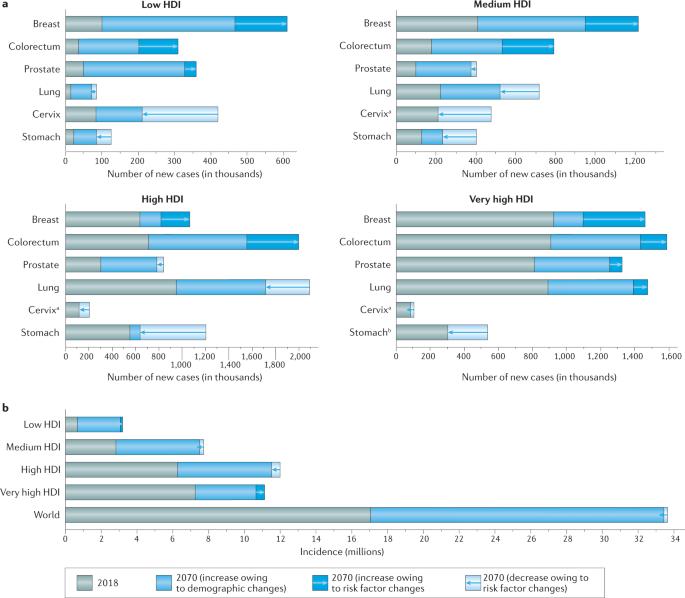
“Assuming that the latest incidence trends continue for the major cancer types, the article in Nature Reviews predicts a doubling of the incidence of all cancers combined by 2070 relative to 2020.
- Focusing on an in-depth assessment of prevention strategies that target tobacco smoking, overweight and obesity, and human papillomavirus infection, we discuss how stepwise, population-level approaches with amenable goals can avert millions of future cancer diagnoses worldwide.
- In the absence of a step-change in cancer prevention delivery, tobacco smoking will remain the leading preventable cause of cancer, and overweight and obesity might well present a comparable opportunity for prevention, given its increasing prevalence globally in the past few decades.
- Countries must therefore instigate national cancer control programmes aimed at preventing cancer, and with some urgency, if such programmes are to yield the desired public health and economic benefits in this century.”
Proceed to the article.
Source: Vivek Subbiah/LinkedIn
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research at the MD Anderson Cancer Network and a former Associate Professor in the Department of Investigational Cancer Therapeutics at The University of Texas MD Anderson Cancer Center.
Dr. Vivek Subbiah has served as the principal investigator in over 100 phase I/II trials and co-investigator in over 200 clinical trials and is known for his leadership in several first-in-human and practice-changing studies that directly led to approvals from the FDA, European Medicines Agency, and other agencies across the world. He is an expert in tumor agnostic precision oncology and leads the BRAF and RET tissue agnostic studies to FDA approval.


