Marco Donia, Associate Professor at National Center for Cancer Immune Therapy, Denmark, shared on LinkedIn:
“Adjuvant Anti-PD-1 Immunotherapy for Resected Melanoma: Update December 2023
Reduces relapses by ~40% (RFS and DMFS, HR ~0.5-0.6) in resected stage ≥IIB.
~15% serious (≥grade 3) irAE, almost 30% chronic irAE [previous refs]
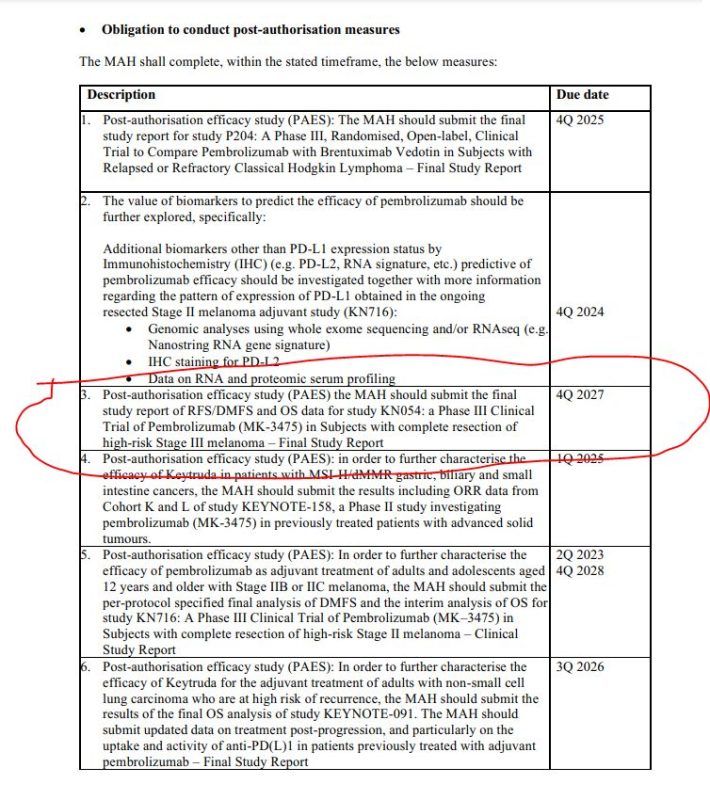
No OS data; could be delayed until END 2027.
New summary of product characteristics for Pembrolizumab by the European Medicines Agency (update October 2023) changed the date for submission of final OS analysis to the end of 2027.
This is +11 years after the last patient was enrolled in the original phase III trial of Pembrolizumab (KEYNOTE-054) as adjuvant treatment for patients with resected stage III melanoma. ‘the protocol-specified number of deaths (380) had not yet been reached (2022), no overall survival analysis was conducted at the time of the current analysis’ (NEJM Evidence)
My take home:
- Reducing the risk of relapse is a critical outcome.
- However, there is a non-negligible risk of important side effects and the possibility of administering a toxic drug to patients potentially cured by surgery.
- To provide high-quality information to patients, it would be highly desirable to understand whether anti-PD-1 immunotherapy given upfront (adjuvant, after resection) improves the survival of patients with resected high-risk melanoma.
(While the scientific reasons not to perform an OS analysis before the pre-defined number of ‘events’ should be recognized, the inability to provide information on the rate of 7-year survival to patients before initiation of adjuvant immunotherapy is a critical issue in Dec 2023.)
Adjuvant immunotherapy is a type of cancer treatment given after the primary treatment, such as surgery, to reduce the risk of cancer returning by destroying any potentially remaining cancer cells. It works by boosting the immune system to identify and destroy cancer cells.
Glossary:
RFS: Relapse-free Survival
DMFS: Distant metastasis-free Survival
OS: Overall Survival
irAE: Immune-related adverse events
This post will be updated if new information becomes available – please share additional information in the comments.”
Read further in
See more about OS analysis.
Source: Marco Donia/LinkedIn


