Nina Niu Sanford, Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital, shared on X:
“RTOG 1112 finally out! Sorafenib +/- SBRT in HCC.
Congrats to Laura Dawson and team.
Brief summary here and some of my thoughts.
This was a Phase III RCT of sorafenib +/- SBRT in Stage B/C HCC not amenable to resection, ablation, TACE.
N=193.
These were advanced tumors. In addition to not being eligible for other local therapies, 74% had MVI & median tumor size was 7.8 cm.
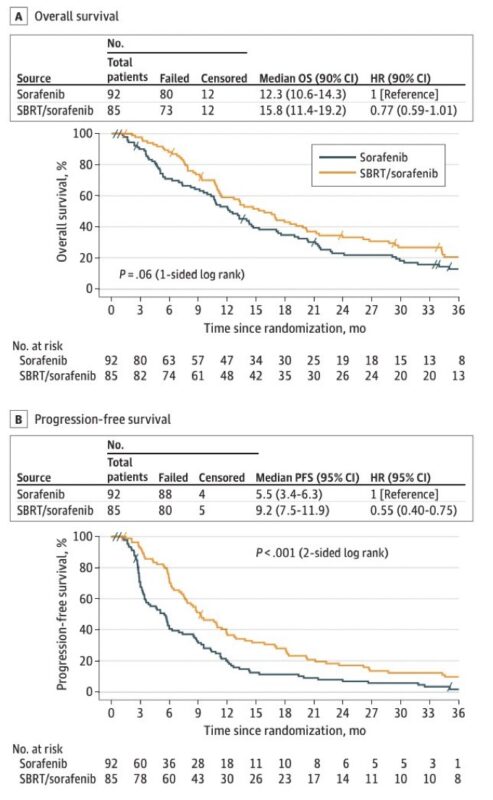
OS was numerically better with SBRT (15.8 vs. 12.3 months) though not significant (1-sided p=0.06).
PFS was improved 5.5 to 9.2 months (p= <0.001), as was time to progression.
The trial was closed early due to slow accrual/new SOC systemic.
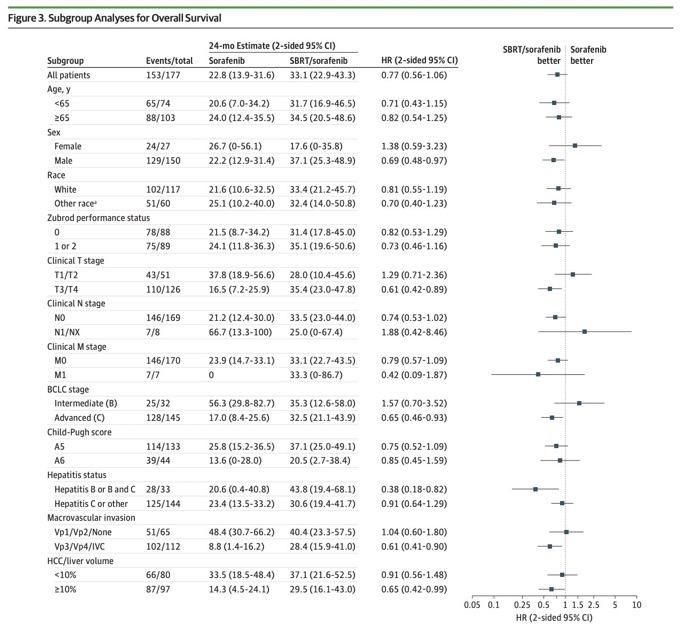
In subgroup analysis for more advanced tumors (VP3/VP4/IVC), OS was significantly improved with addition of SBRT (see bottom of forest plot).
Sidenote, not to beat a dead horse (but I will be happy), why was primary endpoint here OS but in say TACE trials (LEAP, EMERALD) it’s PFS? If RTOG 1112 had PFS as 1 endpoint, would be ‘positive.’
OS arguably right endpoint in diseases with poor prognoses, but why the discrepancy?
Anyway, in terms of toxicity, SBRT in this trial added nothing measurable (no differences in Grade 3+ AE between arms).
Quality of life was also better with SBRT, however the number of participants, who filled out these surveys were low, so I think this data point is less reliable.
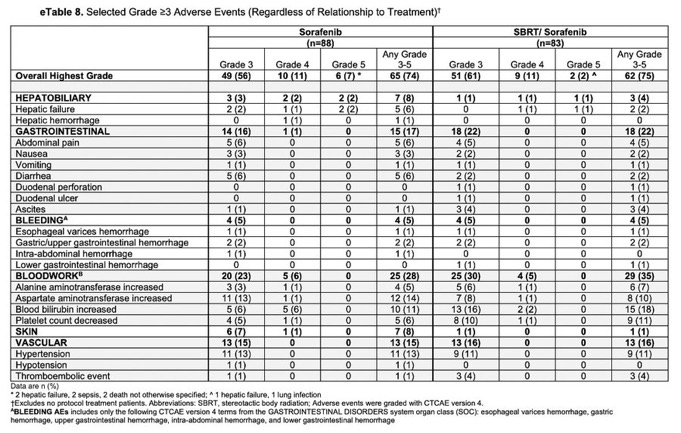
Major limitation is comparison arm is sorafenib, which we know has low response rates, but was SOC when trial was started.
FWIW, most second line drugs in HCC approved based on trials where control was sorafenib.
Would SBRT have additional benefit on top of atezo/bevo or durva/tremi?
I believe yes, but this exact q will be tested in upcoming NRG GI012 RCT – which focuses on more advanced VP3/4 tumors given suggestion of additional benefit to local therapy in RTOG 1112. PI Jen Wo.

So, what can we conclude from RTOG 1112?
Is SBRT+sorafenib a curative treatment for Stage C HCC? No.
Even so and despite study limitations, I think findings provides evidence supporting use of SBRT – a low toxicity and convenient treatment – in HCC, esp large tumors with MVI.
Nina Niu Sanford is an Assistant Professor and Chief of Gastrointestinal Radiation Oncology at Harvard/Brigham and Women’s Hospital/Massachusetts General Hospital. She specializes in treating gastrointestinal cancers and actively participates in clinical trials combining high-dose radiation therapy with immunotherapy. Additionally, she researches healthcare access disparities and conducts pan-cancer outcomes research using large databases.