Michelle Monje, Professor of Pediatric Neuro-Oncology in the Department of Neurology and Neurological Sciences at Stanford University and a Howard Hughes Medical Institute Investigator, shared a recent article on X:
“This is the next chapter of a story about courageous patients and their families, of multidisciplinary teamwork, and hard-fought steps forward to effective therapy for DIPG and DMG, a universally lethal cancer of the brain and spinal cord.
Title: Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas
Authors: Michelle Monje, Jasia Mahdi, Robbie Majzner, Kristen W. Yeom, Liora M. Schultz, Rebecca M. Richards, Valentin Barsan, Kun-Wei Song, Jen Kamens, Christina Baggott, Michael Kunicki, Skyler P. Rietberg, Alexandria Sung Lim, Agnes Reschke, Sharon Mavroukakis, Emily Egeler, Jennifer Moon, Shabnum Patel, Harshini Chinnasamy, Courtney Erickson, Ashley Jacobs, Allison K. Duh, Ramya Tunuguntla, Dorota Danuta Klysz, Carley Fowler, Sean Green, Barbara Beebe, Casey Carr, Michelle Fujimoto, Annie Kathleen Brown, Ann-Louise G. Petersen, Catherine McIntyre, Aman Siddiqui, Nadia Lepori-Bui, Katlin Villar, Kymhuynh Pham, Rachel Bove, Eric Musa, Warren D. Reynolds, Adam Kuo, Snehit Prabhu, Lindsey Rasmussen, Timothy T. Cornell, Sonia Partap, Paul G. Fisher, Cynthia J. Campen, Gerald Grant, Laura Prolo, Xiaobu Ye, Bita Sahaf, Kara L. Davis, Steven A. Feldman, Sneha Ramakrishna and Crystal Mackall.

We report the final results of the first arm of our clinical trial using GD2-CAR T cells to treat children and young adults with DIPG and spinal cord DMG.
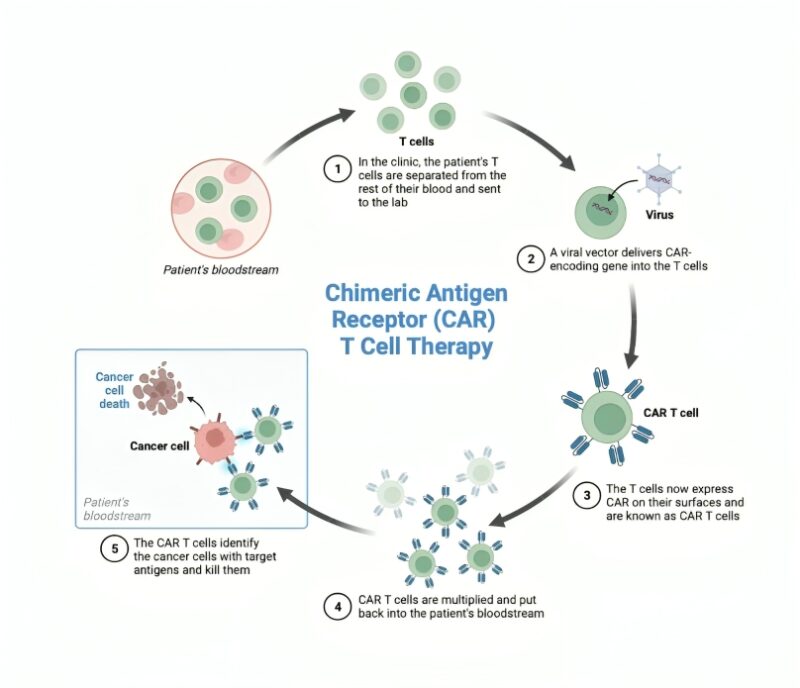
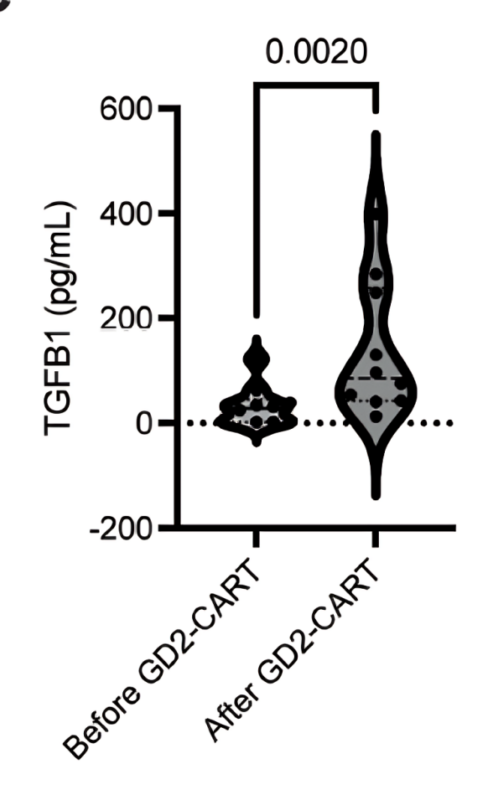
GD2-CAR T cells were delivered intravenously (IV) after a standard preparatory regimen of chemotherapy, and patients who showed benefit from IV CAR T cell therapy were eligible for subsequent CAR T cell doses delivered intracranially into the lateral ventricles through a sort of “port” called an Ommaya reservoir.
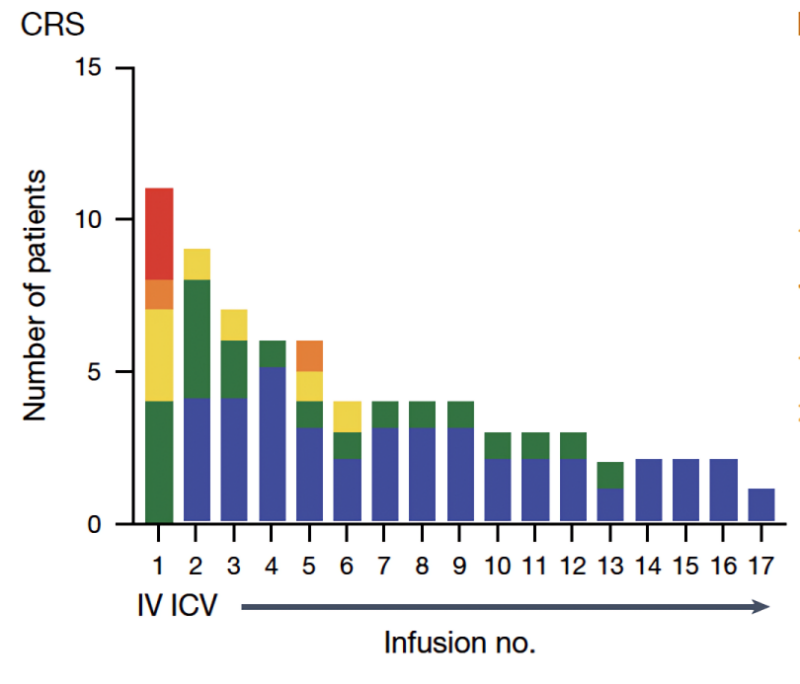
We found that high-grade Cytokine Release Syndrome (CRS) was dose-limiting after IV therapy, but CRS was not a problem after intracranial delivery of CAR T cells.
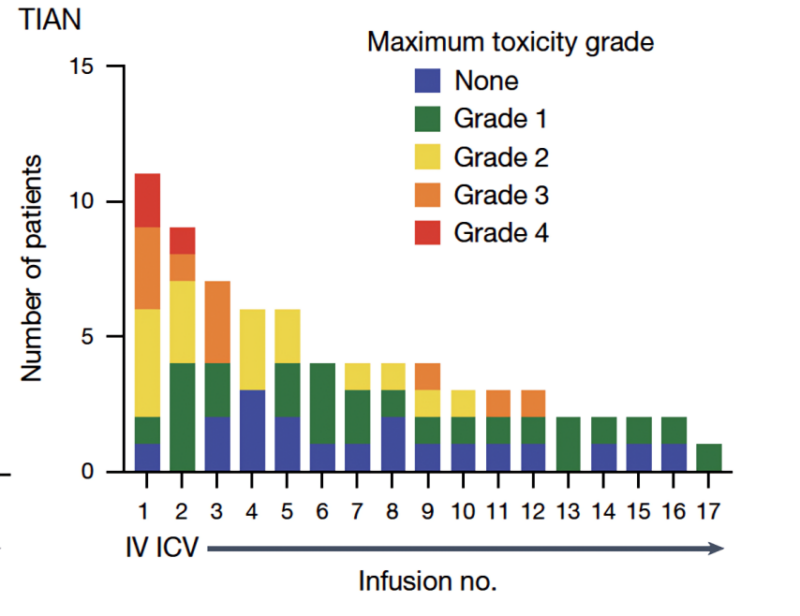
Tumor inflammation-associated neurotoxicity – neurological symptoms caused by therapeutic inflammation in the location of the tumor, was a major category of side effects, and supporting patients through periods of therapeutic inflammation in critical neural structures like the brainstem requires careful, multidisciplinary and often intensive care.
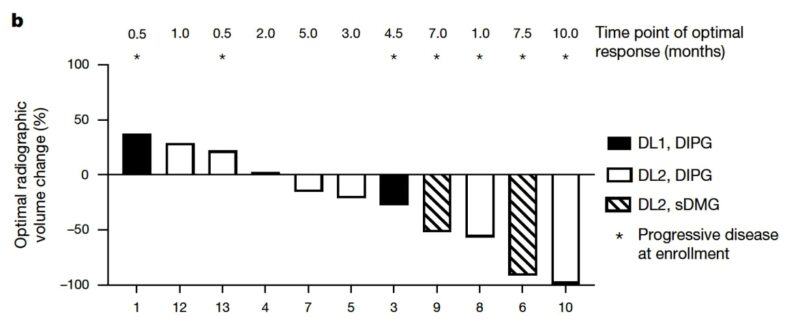
We treated 11 patients on trial, 9 of whom experienced neurological improvements after IV CAR T cell therapy and went on to receive further intracranial doses of CAR T cells. Seven of the 11 patients showed overall shrinkage of their tumors, 4 of whom had overall reductions in tumor volume over 50%. The tumor responses fit a bell-shaped curve (Gaussian distribution). From the perspective of change in tumor size, there were no outliers.
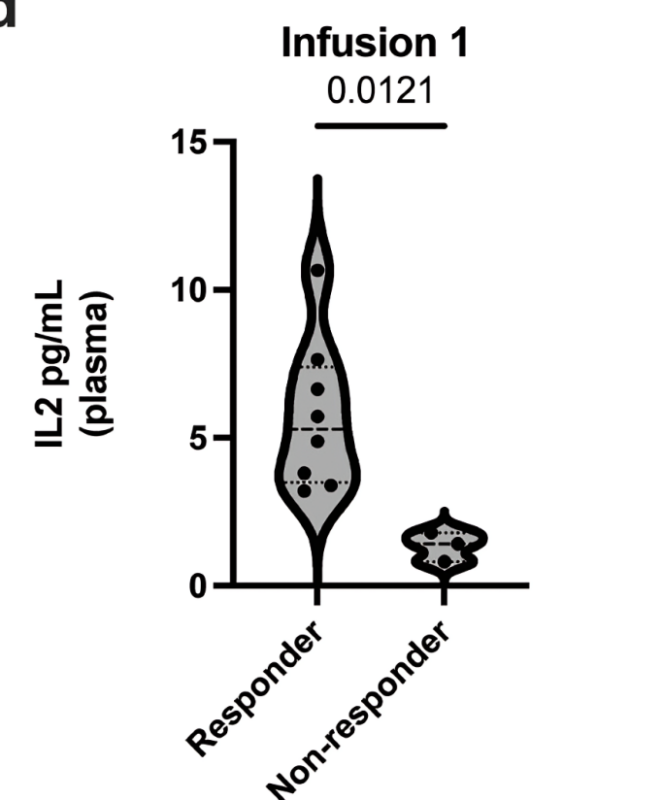
Correlative studies of cells, ctDNA and cytokines evident in CSF before and during CAR T cell therapy provide some explanation for differences in therapeutic efficacy for each patient. Correlatives led by Sneha Ramakrishna with Bita Sahaf.
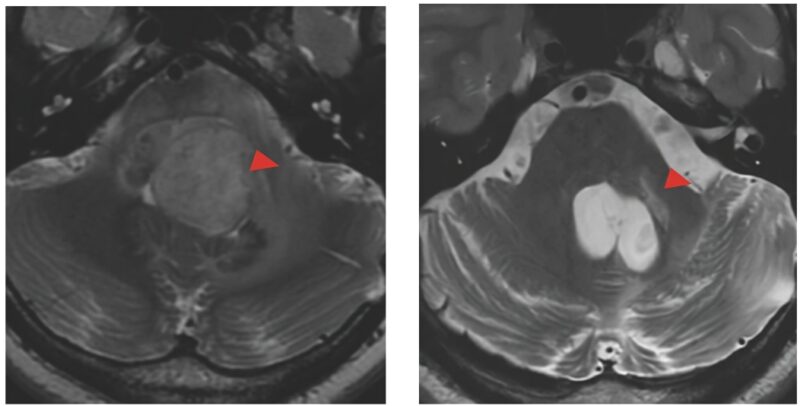
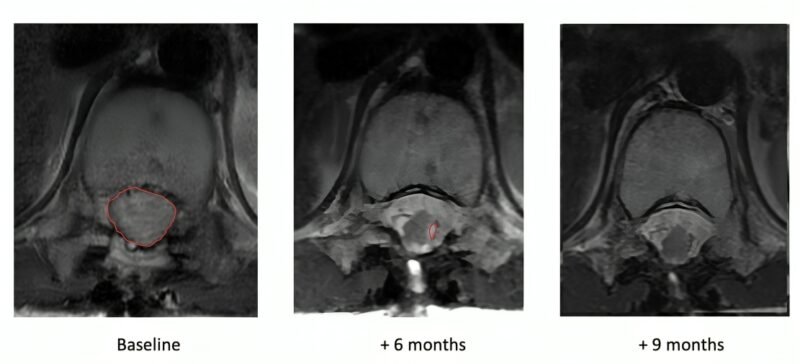
One teenager with DIPG has had a complete response, disappearance of a large tumor that is still gone, now over 3 years from beginning therapy.
This is a story of hope, and the heroism of these patients and their families. I believe that this CAR T cell strategy will one day be central to the cure for DIPG and DMG, and that we will get there in part because of the gifts of knowledge that these pioneering patients have left us.
This clinical trial represents a wonderfully collaborative team effort co-led together with Crystal Mackall and executed with co-PIs Jasia Mahdi and Robbie Mazjner, Majzner_Lab and colleagues in pediatric oncology, neurology at Stanford Neurology and Neurological Sciences, neuro-oncology, neurosurgery at Stanford Neurosurgery.
Critical care and more colleagues from the Center for Cancer Cellular Therapy at Stanford Center for Cancer Cell Therapy, stanfordchildrens, including Liora Schultz, Cynthia Campen, Kun Wei Song, Gerry, Shabnum Patel, Kara Davis and many more.
Grateful to funding for this work from National Cancer Institute, Cure Search, California Institute for Regenerative Medicine, St. Baldrick’s Foundation, Alex’s Lemonade Stand Foundation, Chad Tough Defeat DIPG Foundation, Parker Institute for Cancer Immunotherapy, Ludwig Cancer, Oscars Kids America and others.
For more updates, follow OncoDaily.


