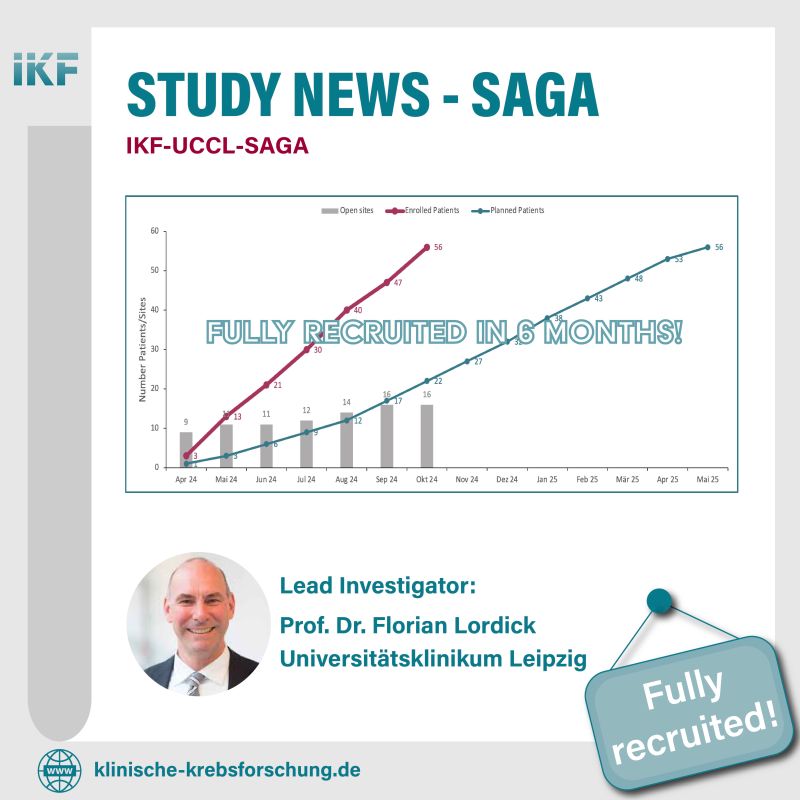
The SAGA Trial has successfully completed full recruitment in just six months, marking a significant milestone in cancer research.

This remarkable achievement highlights the dedication and hard work of everyone involved, from study teams to participating sites and investigators.
A special congratulations goes to Florian Lordick, the Oberall Study Lead and Lead Investigator from Universitätsklinikum Leipzig AöR, along with all partner institutions, for their unwavering commitment to advancing cancer research.
Florian Lordick is a Professor of Medicine at the Universität Leipzig. He is the Editor in Chief at ESMO. Previously, he was the President at the International Gastric Cancer Association. He completed her education from the Technical University of Munich.

This study represents a groundbreaking collaboration between UCCL Leipzig and IKF – The Frankfurt Institute of Clinical Cancer Research, setting the stage for many more innovative projects in the future.
The SAGA trial is a Phase Ib/II study evaluating sacituzumab govitecan, a TROP2-targeting antibody, for patients with metastatic esophagogastric adenocarcinoma.
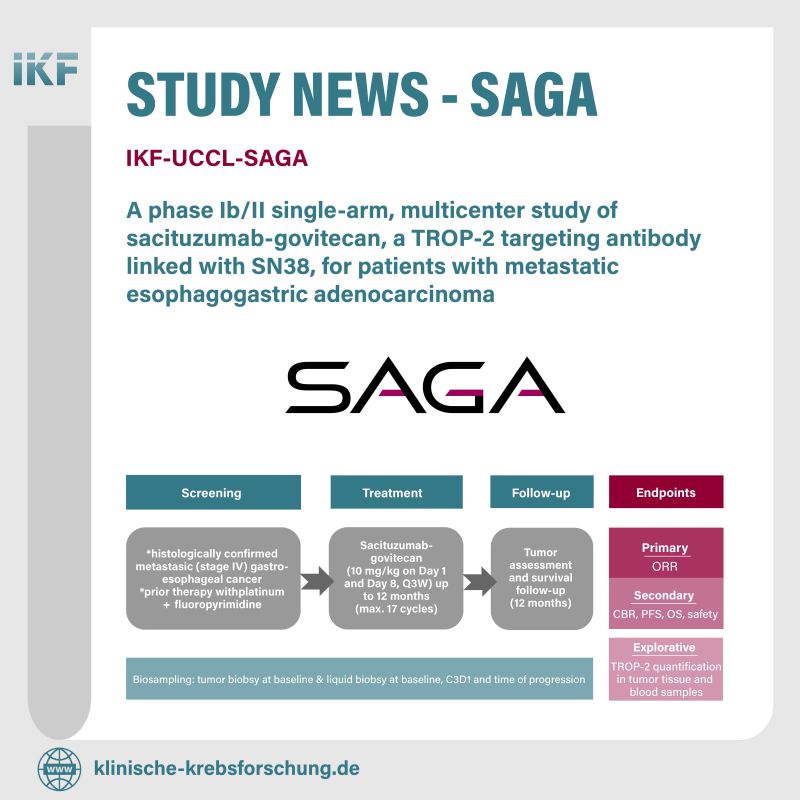
This trial aims to provide new hope for those affected by this challenging condition.
This success underscores a mission to accelerate drug development and improve patient outcomes through investigator-initiated trials and academic drug development.
For more posts like, visit oncodaily.com


