The FDA has approved tumor treating fields (TTFields; Optune Lua) in combination with docetaxel or PD-L1 inhibitors for patients with metastatic non-small cell lung cancer (NSCLC) that has progressed during or after platinum-based therapy.
This landmark decision is supported by data from the phase 3 LUNAR trial (NCT02973789), conducted by Novocure, which revealed substantial improvements in patient outcomes.

Trial Overview and Key Findings
The LUNAR trial aimed to assess the efficacy of TTFields when combined with standard of care (SOC) treatments—specifically, immune checkpoint inhibitors (ICIs) or docetaxel. The study’s design included randomizing eligible patients to receive either TTFields alongside SOC or SOC alone.
Overall Survival (OS)
TTFields + PD-L1 Inhibitors: The trial reported a median overall survival of 19.0 months (95% CI, 10.6-28.2 months) for patients receiving the combination therapy, compared to 10.8 months (95% CI, 8.3-17.6) for those on PD-L1 inhibitors alone (P = .002).
TTFields + Docetaxel: Similarly, patients treated with TTFields and docetaxel had a median OS of 11.1 months (95% CI, 8.2-14.1) versus 8.9 months (95% CI, 6.5-11.3) for those receiving docetaxel alone.
These findings highlight a statistically significant increase in survival for patients treated with TTFields, suggesting that this innovative therapy can enhance the efficacy of existing treatment options.
Progression-Free Survival (PFS)
The trial also evaluated PFS, with the TTFields group achieving a median of 4.8 months (95% CI, 4.1-5.7) compared to 4.1 months (95% CI, 3.1-4.6) in the SOC-only group. Although this difference did not reach statistical significance (P = .23), it indicates a potential trend towards improved outcomes.
Adverse Effects and Safety Profile
The safety profile of TTFields was also assessed in the trial. Overall, adverse events (AEs) occurred in 97% of patients receiving TTFields and SOC, compared to 91% in the SOC alone group. Most reported AEs were grade 1 or 2, primarily consisting of local skin irritations such as dermatitis, pruritus, and rash.
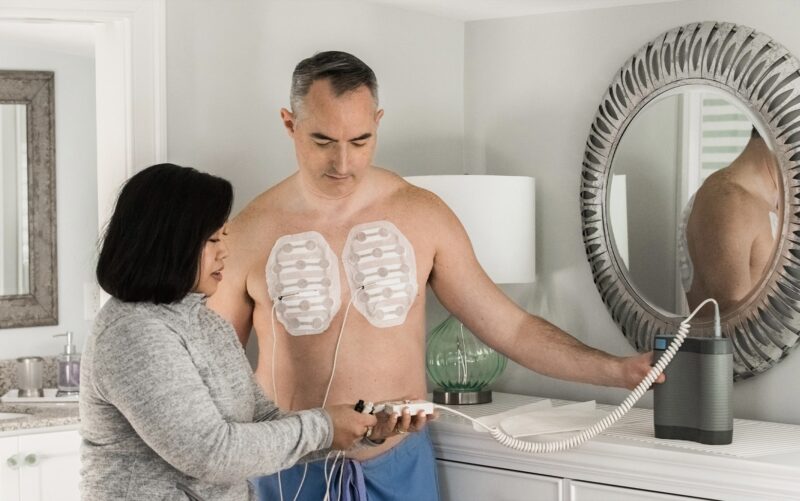
Device-Related AEs: TTFields-related AEs were reported in 71% of the treatment group. Notably, 12% of patients in the TTFields arm required dose reductions of standard therapy, and 14% discontinued due to device-related toxicity.
Despite the incidence of AEs, the localized nature of the side effects contrasts with the systemic toxicity often seen with traditional chemotherapy and immunotherapy, making TTFields an appealing option for many patients.
Expert Perspective
Ticiana Leal, the primary investigator and director of the Thoracic Oncology Program at Emory University, underscored the importance of these findings.
“There have been a number of important advances in first-line treatment for NSCLC, but this is an aggressive disease, and most patients will develop progression, with limited effective treatment options in the second line and beyond, The OS results we observed with TTFields in the LUNAR study mark the first substantial improvement in more than 8 years in this patient population, making this a compelling development for many patients and their physicians.”
Conclusion
The approval of TTFields for advanced NSCLC represents a critical breakthrough in treatment options for patients facing limited alternatives. With the potential for improved survival and a favorable safety profile, TTFields could change the landscape of therapy for this challenging disease.
For more posts like this, visit oncodaily.com