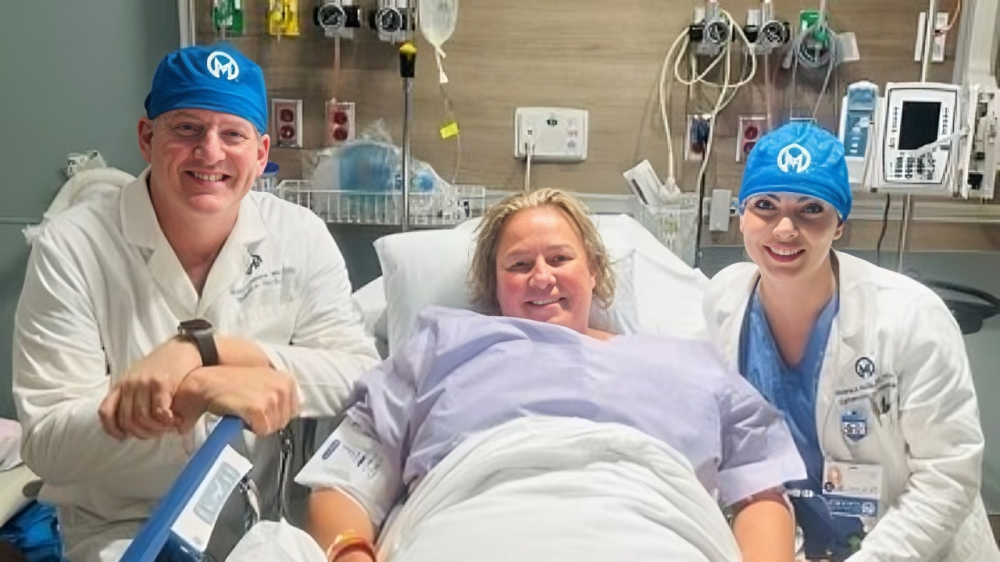
Anixa Biosciences, Inc., a biotechnology firm focused on cancer treatment and prevention, has announced the administration of a second dose of its CAR-T therapy to a patient.
This follows a positive response to the initial treatment in the ongoing Phase 1 clinical trial of its chimeric antigen receptor T-cell (CAR-T) therapy for ovarian cancer, conducted in partnership with Moffitt Cancer Center.

Anixa and Moffitt received approval for a single-patient IND application, allowing the second dose to be given to a patient whose tumor biopsy indicated cellular infiltration and necrosis, showing the CAR-T therapy’s biological activity.
After the first infusion, the patient remained stable, did not require alternative treatment, and reported a good quality of life. These results suggest that CAR-T therapy may serve as a successful long-term treatment option for ovarian cancer.
“Although this is a single patient, the clinical activity observed, including necrosis and T cell infiltration, suggests the therapy’s potential effectiveness,” stated Dr. Robert Wenham, Chair of the Department of Gynecologic Oncology at Moffitt and the trial’s principal investigator. He noted that an amendment has been submitted to allow patients who may benefit to receive a second dose and expressed eagerness to continue evaluating this treatment.

Dr. Monica Avila, the patient’s oncologist, noted that the patient received her first infusion in May 2023. Nearly 18 months later, she is doing well and is now receiving a second dose.

Dr. Amit Kumar, CEO of Anixa Biosciences, emphasized the team’s commitment to advancing this innovative therapy, noting that the ongoing clinical improvements reinforce their confidence in the treatment’s potential to provide hope for ovarian cancer patients.

The Phase 1 clinical trial at Moffitt is focusing on recurrent ovarian cancer patients who have not responded to standard-of-care therapies. To date, six patients have been treated in the dose escalation trial, with three in the first cohort and three in the second cohort. Dose escalation will continue after confirming the safety of previous dosages.
Anixa Biosciences shared a post on LinkedIn about the news:
“Anixa has administered the second dose of its CAR-T therapy to a patient following a positive response in its Phase 1 ovarian cancer trial, conducted in collaboration with Moffitt Cancer Center.
Overview of Ovarian Cancer
Ovarian cancer is primarily classified into three types: epithelial tumors, germ cell tumors, and sex-cord stromal tumors, with epithelial ovarian cancer (EOC) being the most common, accounting for 85-90% of cases. The disease often arises from genetic mutations, particularly in BRCA1 and BRCA2 genes, and its pathogenesis involves a complex interplay of genetic, environmental, and hormonal factors. Due to the lack of definitive early symptoms, many patients are diagnosed at advanced stages, leading to a high mortality rate.
Risk Factors and Clinical Presentation
Key risk factors include age (most commonly diagnosed in women over 50), family history of breast or ovarian cancer, nulliparity, and obesity. Symptoms are often vague and may include abdominal bloating, pelvic pain, and urinary urgency, which can delay diagnosis. Standard diagnostic methods involve imaging and serum biomarker tests like CA-125. Treatment typically includes surgical debulking followed by chemotherapy, with early detection being critical for improving patient outcomes.
Immunotherapy for Ovarian Cancer: A Growing Field