George Pentheroudakis, Chief Medical Officer at ESMO, shared the following post by Vivek Subbiah on X, adding the following:
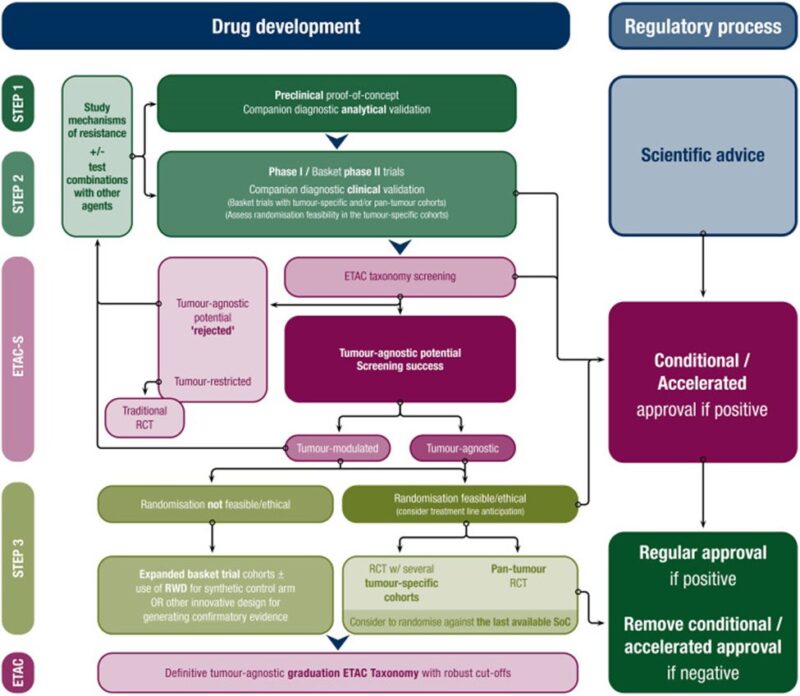
“Following a drug screening positive by ETAC-S, we propose a research and regulatory framework for validation/graduation of tumour-agnostic therapies and biomarkers.”
Quoting Vivek Subbiah’s post:
“Proposed tumour-agnostic drug development framework considering the deployment of efficient modern trial designs and streamlined regulatory processes which can exploit targeted driver molecular aberrations across tumour types.”
Read Vivek Subbiah’s thread in its entirety for additional insights.
Source: George Pentheroudakis/X and Vivek Subbiah/X
Vivek Subbiah is the Chief of Early-Phase Drug Development at the Sarah Cannon Research Institute (USA). He is the former Executive Director of Oncology Research and former Associate Professor in the Department of Investigational Cancer Therapeutics at the MD Anderson Cancer.
He focuses on translational cancer research and the design and implementation of early-phase biomarker-driven clinical trials. His work specifically targets antibody-drug conjugates, radiopharmaceuticals, immunoconjugates, and basket trials.


