Quoting Shankar Siva, Radiation Oncologist at the Peter MacCallum Cancer Centre, on X/Twitter:
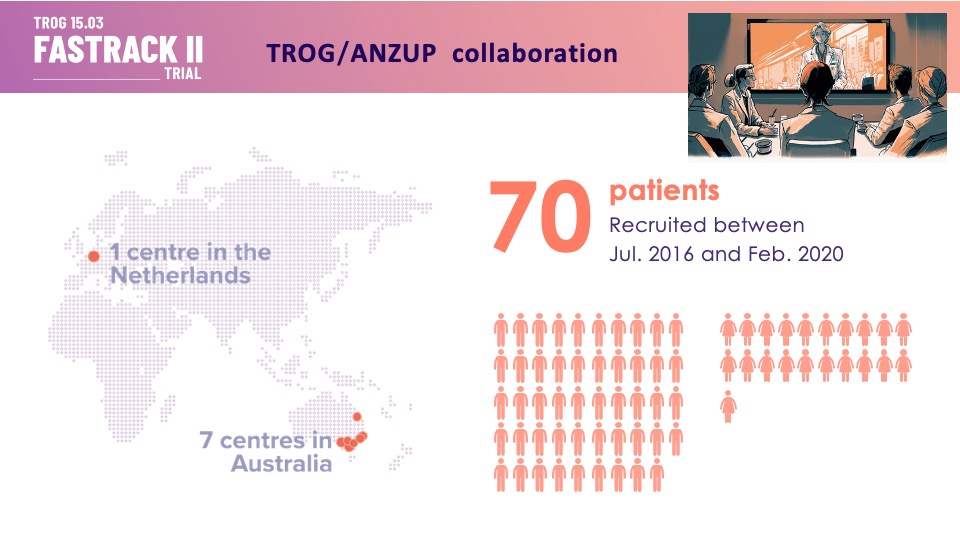
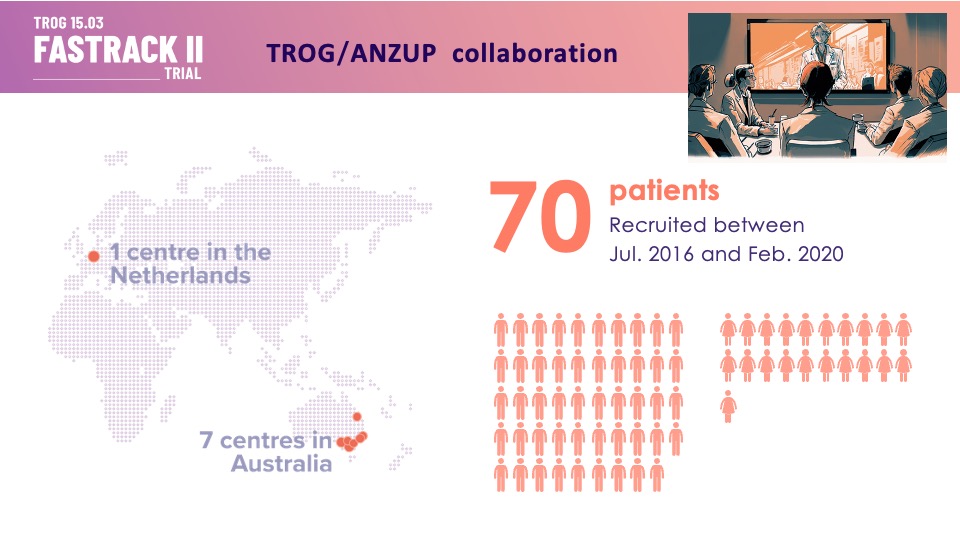
“Thank you patients, families, trial staff and investigators for participating in TROG (Trans Tasman Radiation Oncology Group) Cancer Research 15.03 FASTRACK II! This trial recruited 70 people across 8 sites in Australia and the Netherlands.
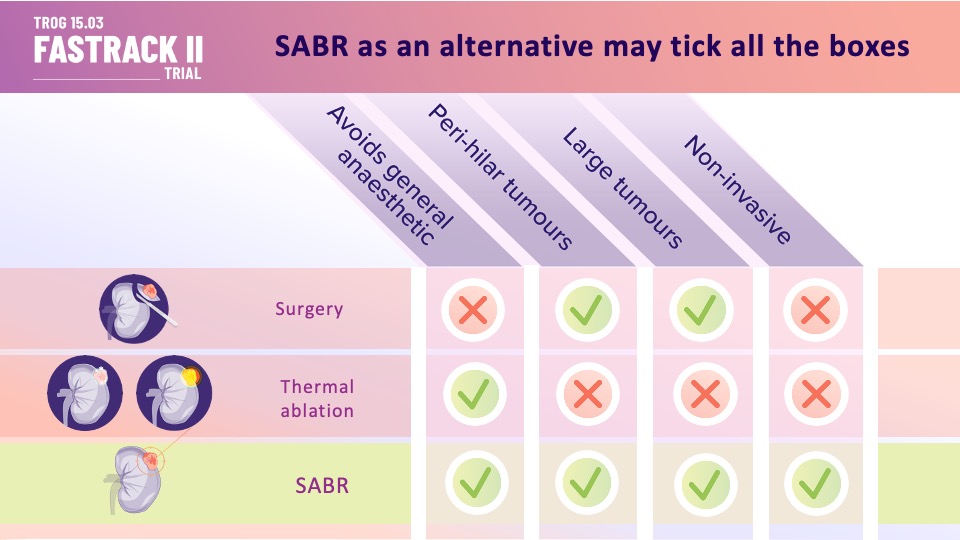
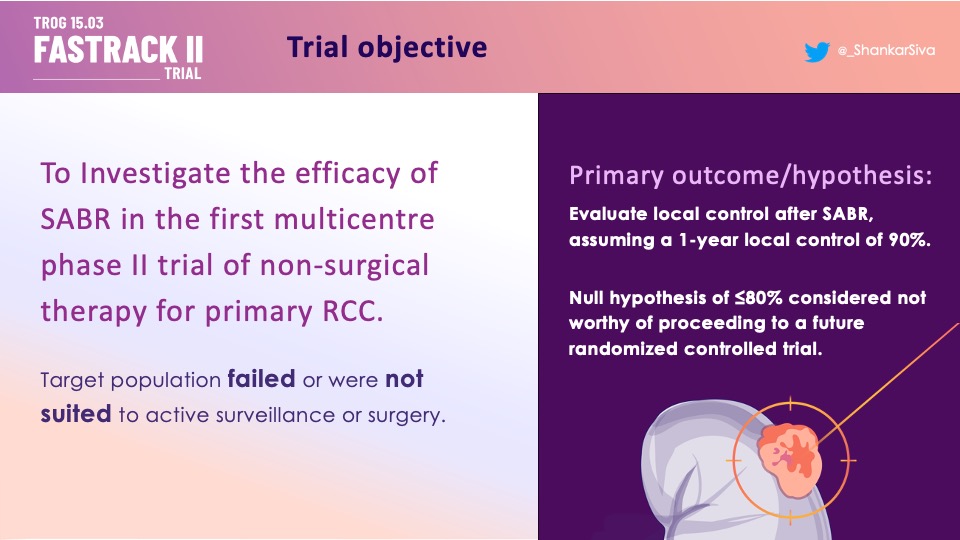
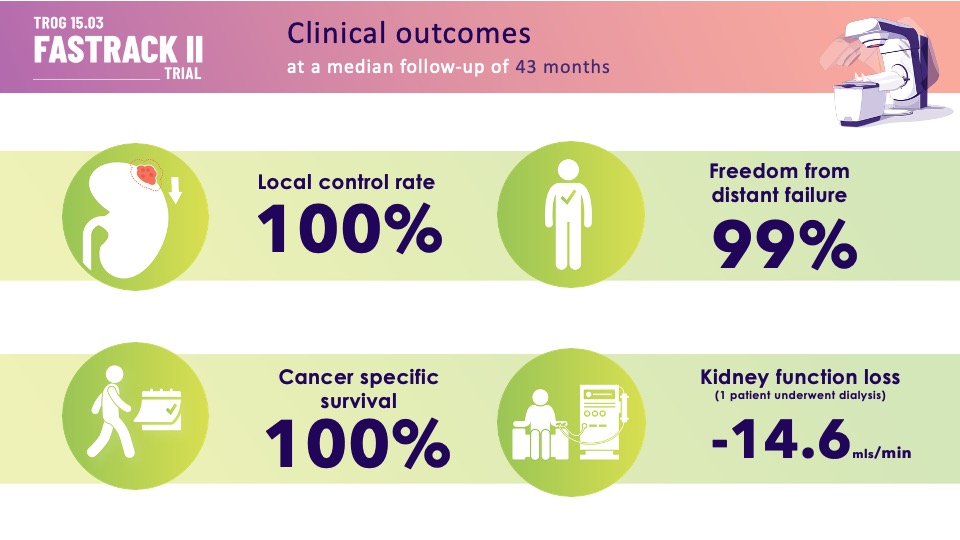
SABR (Stereotactic Ablative Body Radiotherapy) is a novel treatment for primary kidney cancer which is non-invasive and has fewer technical limitations than thermal ablation. We tested SABR in an Australian and the Netherlands phase II trial for primary RCC (Renal Cell Carcinoma) that was inoperable or at high risk for surgery. The benchmark was 90% control at 1-year.
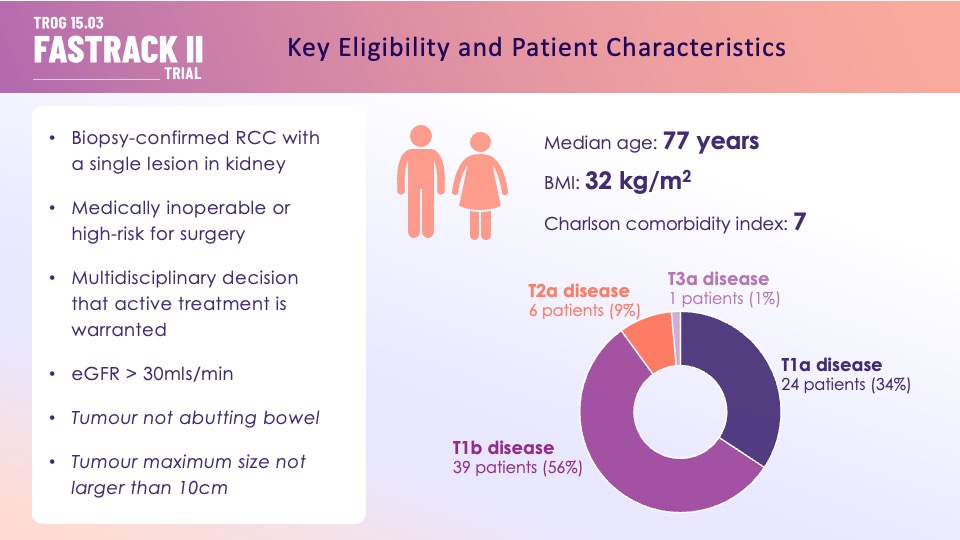
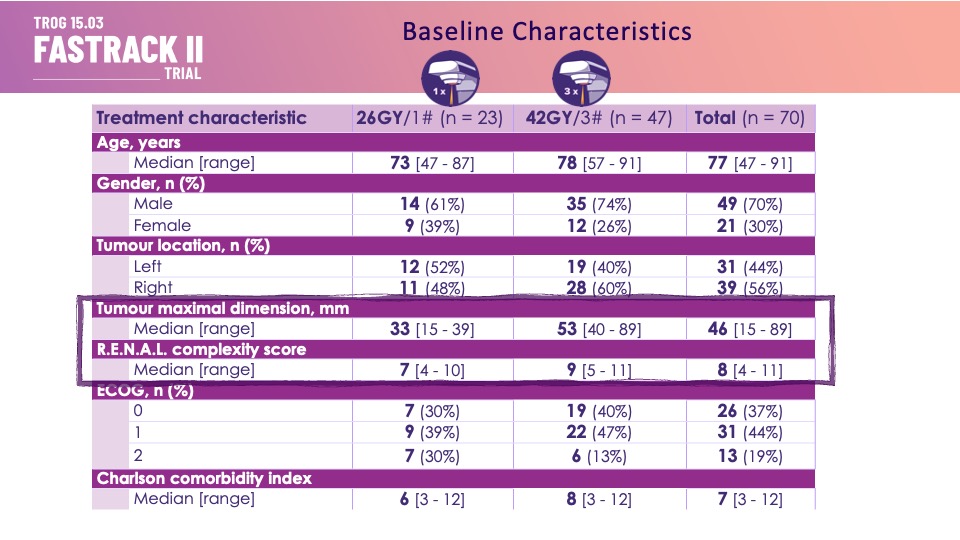
Eligible patients had an MDM (Medical Decision Making) recommendation for active treatment, 100% biopsy confirmed, eGFR>30. ~1/3 had T1a disease, most had larger (median size 46mm, largest 89mm). renal complexity ~8, n=70.
Tumor size decreased than randomized controlled trials of Registered Nurse vs. Nurse Practitioner. Toxicity acceptable. As yet, there are no published clinical trials of thermal ablation; therefore, SABR is now a acceptable standard of care for inoperable Renal Cell Carcinoma. FASTRACK II findings should lead to an randomized controlled trials vs surgery.”
Source: Shankar Siva/Twitter