Recently, an article by Srinivas Viswanathan et al. titled “A genetic basis for sex differences in Xp11 translocation renal cell carcinoma” was published in Cell.
Authors: Mingkee Achom, Ananthan Sadagopan, Chunyang Bao, Fiona McBride, Jiao Li, Prathyusha Konda, Richard Tourdot, Qingru Xu, Maria Nakhoul, Daniel Gallant, Usman Ali Ahmed, Jillian O’Toole, Dory Freeman, Gwo-Shu Mary Lee, Jonathan Hecht, Eric Kauffman, David Einstein, Toni Choueiri, Cheng-Zhong Zhang, Srinivas Viswanathan.

Srinivas Viswanathan shared a post on X about the paper, adding:
“Our study on sex differences in Xp11 translocation renal cell carcinoma is out in Cell! We ascribe a genetic basis to the female bias in tRCC, which is driven by a translocation of the TFE3 gene on the X chromosome. With Zhang lab.
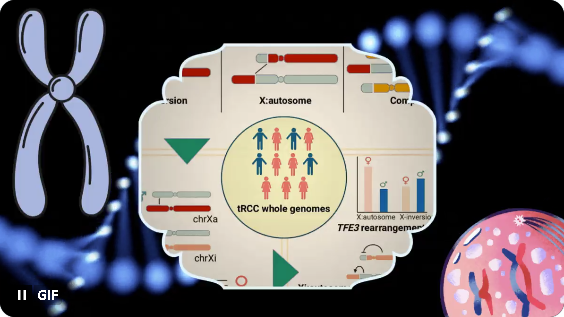
Many cancers show sex differences in incidence but most are male-biased. tRCC, however, is more common in females. As females have twice as many chrX as males, could female sex bias in tRCC have a genetic basis?
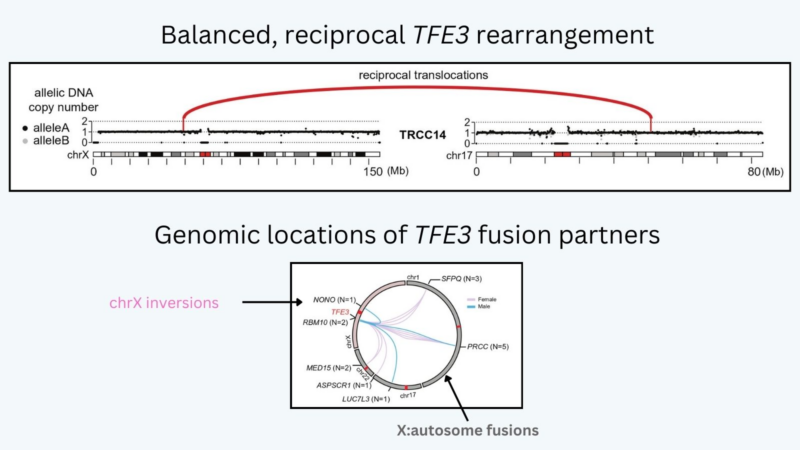
We performed WGS of a tRCC cohort and re-analysis of >100 WES cases. Most tRCC genomes were quiet with balanced and reciprocal TFE3 rearrangements. Multiple TFE3 partner genes were identified, both on autosomes and on chrX.
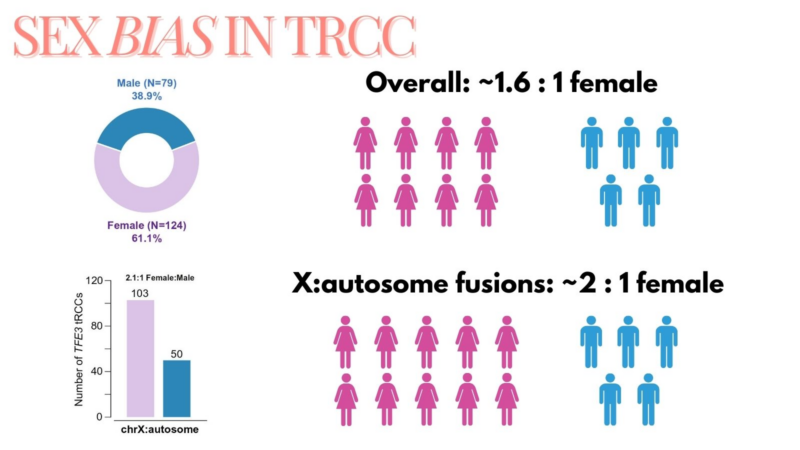
Strikingly, the TFE3 fusions arising via X:autosome translocation (but not X-inversion) have an almost exact 2:1 female:male ratio, explaining the female predominance of tRCC.
While females have two chrX, one homolog (Xi) is largely silenced via X-chromosome inactivation and most transcription occurs from the other homolog (Xa), this achieves dosage compensation. What are the consequences for somatic Xa vs. Xi rearrangements in cancer?
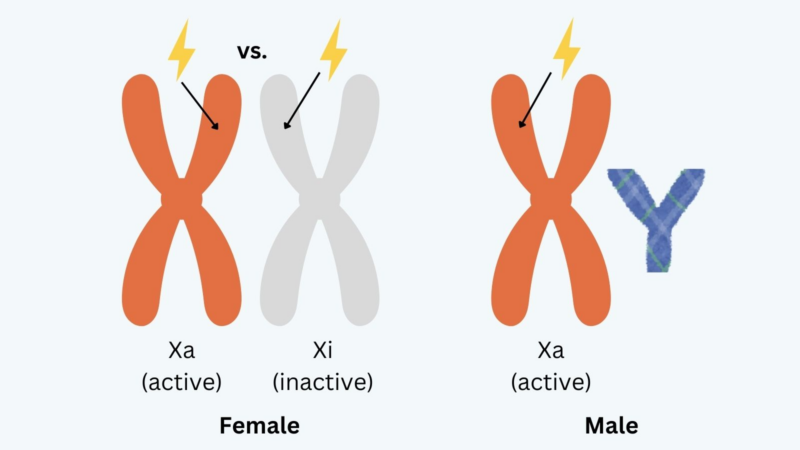
We reasoned that there would be sex-specific constraints and consequences of TFE3 rearrangements. For ex: large losses on Xi may be tolerated due to minimal transcription from this homolog, but would not be tolerated on Xa, from which many essential genes are transcribed.
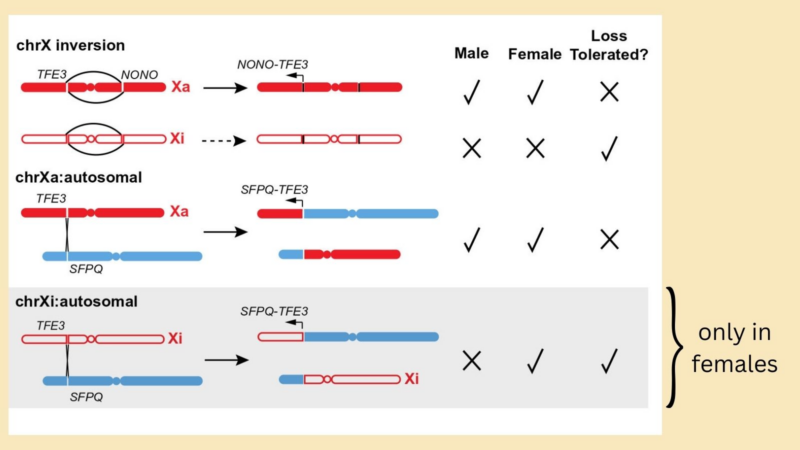
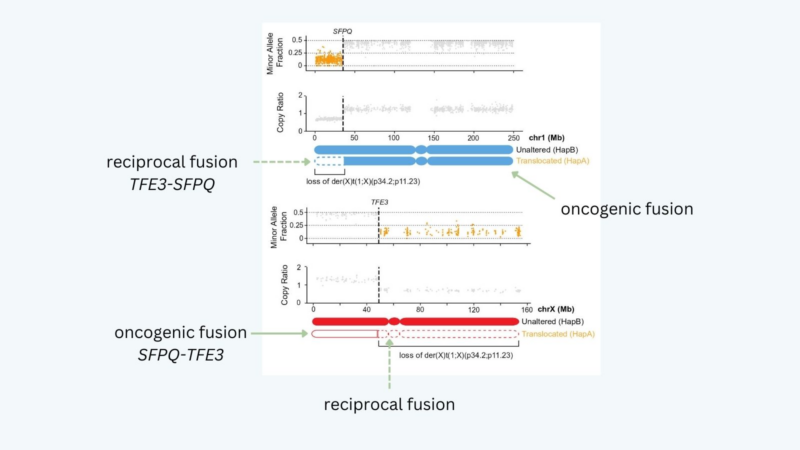
Indeed, we saw deletions of the reciprocal TFE3 fusion chr (which contains most of chrX but does not encode the oncogenic fusion) in females but never in males: these deletions must have occurred on Xi, implying that the Xi can be accessed for X: autosome TFE3 translocations!
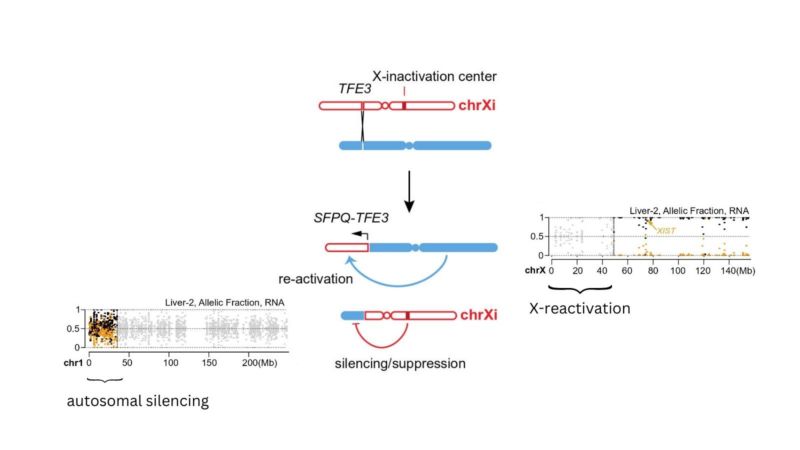
Xi rearrangements may also lead to somatic reactivation of Xi, since translocations can dissociate chrX regions from the X-inactivation center; or autosomal silencing when autosomal regions are translocated near the XIC. We observed these phenomena in tRCC!
Much more to be done to further understand the somatic plasticity of XCI in cancer, genetic determinants of cancer sex bias, and to move towards sex-specific precision oncology. See also a more detailed thread from our preprint if interested.
This was true team science and only possible because of close collaboration with C-Z Zhang lab, talented co-first authors Mingkee Achom, Ananthan Sadagopan, Chunyang Bao and coauthors.
Lastly, grateful to funding sources Damon Runyon Cancer Research Foundation, Doris Duke Foundation, NCI Division of Cancer Biology, CDMRP and to editor Dr. Rita Cha and reviewers for a thorough review that greatly improved the manuscript.
Update: And here is the full text link”
Doctors have been commenting about the article.
“First oncogene discovered from the inactive X chromosome. What a target for cancer drug discovery!”
“Congrats Srinivas Viswanathan, Toni Choueiri et al. Great work!
TFE3 fusions in Xp11 tRCC, a rare female-predominant cancer, arise from reciprocal X chromosome rearrangements.”
“Our own Dana-Farber Lank Center for Genitourinary Oncology superstar physician-scientist Srinivas Viswanathan and team committed to unlock the mysteries behind this rare kidney cancer subtype: Translocation Renal Cell Carcinoma.”
More posts by Srinivas Viswanathan on oncodaily.com
Srinivas Viswanathan is a Principal Investigator at Dana-Farber Cancer Institute and an Assistant Professor at Harvard Medical School. His research primarily focuses on genitourinary cancers, specifically investigating how changes in the noncoding genome or RNA processing pathways contribute to the development of these diseases.
Yüksel Ürün is a Medical Oncology professor at Ankara University School of Medicine in Turkey. His research focuses on genitourinary cancers, covering epidemiology, diagnosis, biomarkers, meta-analysis, and treatment outcomes. His dedication to patient care and research inspires positive change in the medical field.
Toni Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director of International Strategic Initiatives at Dana-Farber and past President of the Medical Staff at DFCI (2016-2018).


