Clinical Cancer Research shared a post on X about a recent paper by Aditya Bardia et. al, adding:
“Phase 1b Study: Sequential Sacituzumab and Talazoparib for triple-negative breast cancer.”
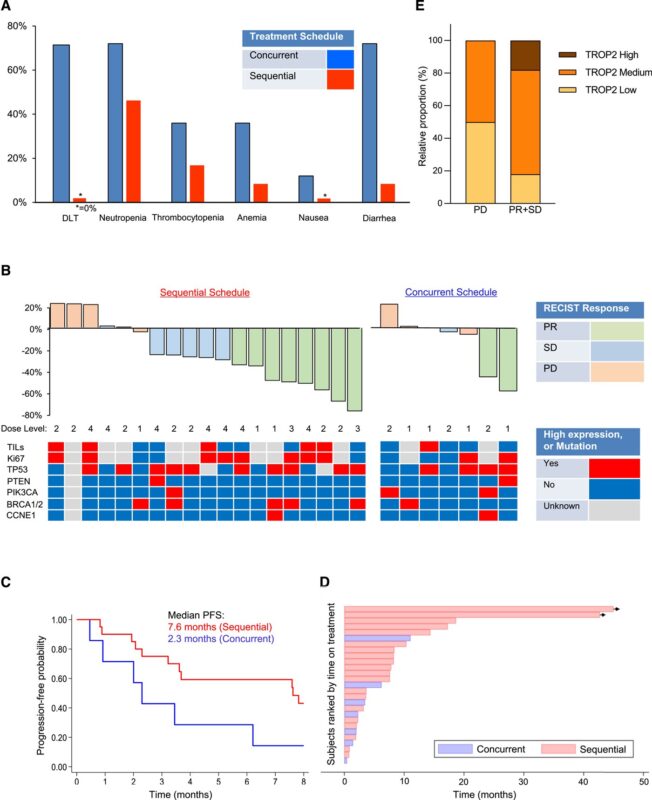
Authors: Aditya Bardia, Sheng Sun, Nayana Thimmiah, James T. Coates, Bogang Wu, Rachel O. Abelman, Laura Spring, Beverly Moy, Phoebe Ryan, Mark N. Melkonyan, Ann Partridge, Dejan Juric, Jeffrey Peppercorn, Heather Parsons, Seth A. Wander, Victoria Attaya, Brenda Lormil, Maria Shellock, Aiko Nagayama, Veerle Bossuyt, Steven J. Isakoff, Sara M. Tolaney, and Leif W. Ellisen.

Raffaele Colombo commented on Clinical Cancer Research’s post on X, adding:
“Sequential sacituzumab govitecan (SG)/talazoparib (TZP) demonstrated median PFS of 7.6 months without DLTs.
Concurrent dosing (combo) of SG and TZP yielded 2.3 months PFS and multiple DLTs including severe myelosuppression.”
Source: Clinical Cancer Research/X and Raffaele Colombo/X
Raffaele Colombo, a leading figure in the pharmaceutical industry is the Associate Director of Medicinal Chemistry at Zymeworks Inc..
His leadership and scientific acumen drive the discovery and optimization of novel drug candidates and advancing the treatment landscape for various diseases.


