Andreas Moor, Assistant Professor of Systems Physiology at ETH Zurich, shared a post on X about a recent paper by Costanza Borrelli et al. titled “In vivo interaction screening reveals liver-derived constraints to metastasis” published in Nature.
Authors: Costanza Borrelli, Morgan Roberts, Davide Eletto, Marie-Didiée Hussherr, Hassan Fazilaty, Tomas Valenta, Atefeh Lafzi, Jonas Kretz, Elena Guido Vinzoni, Andromachi Karakatsani, Srivathsan Adivarahan, Ardian Mannhart, Shoichiro Kimura, Ab Meijs, Farah Baccouche Mhamedi, Ilhan Acar, Kristina Handler, Xenia Ficht, Randall Platt, Salvatore Piscuoglio and Andreas Moor

“How are cell-cell interactions governing successful metastatic colonization in the liver? Have a look at our new paper in Nature that introduces an in vivo proximity screen to functionally test cellular interactions in a high-throughput manner.
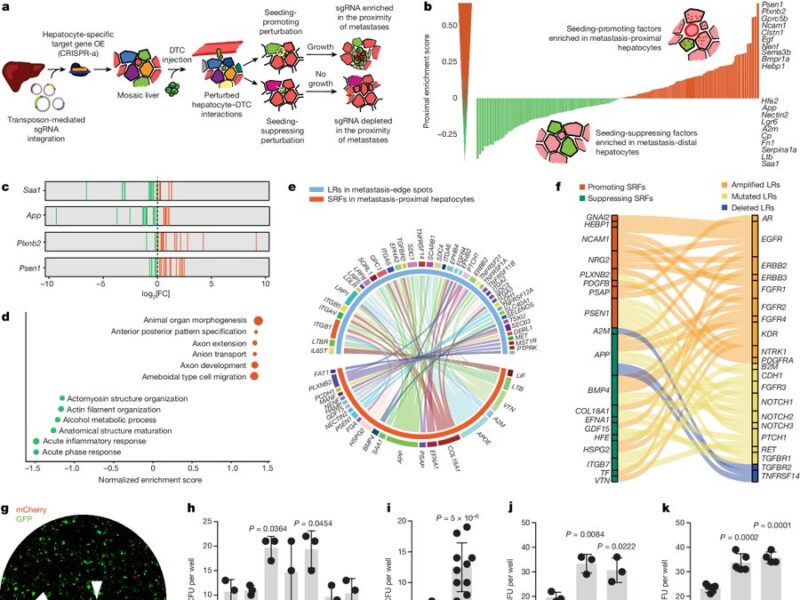
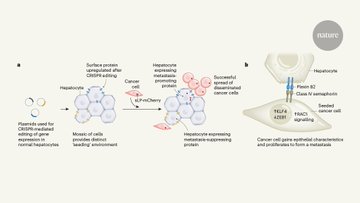
Spearheaded by the amazing Costanza Borrelli, we stably introduced sgRNAs targeting ligands and receptors into hepatocytes of a CRISPR-a mouse to create a mosaic overexpression of these targets. We then induced liver metastasis by intrasplenic injection of cancer organoids.
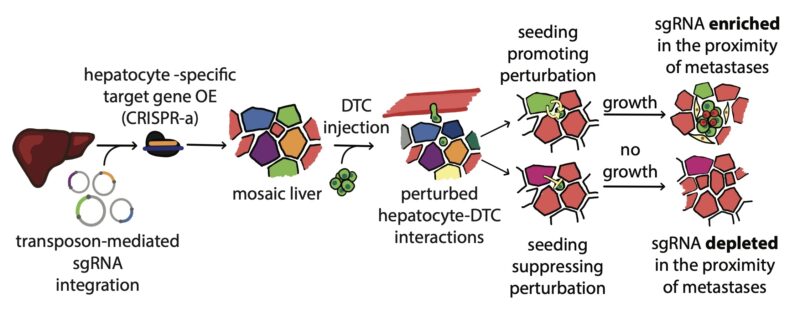
Here you see the natural expression of the Glul gene in one layer of hepatocytes around the central vein. Targeting this gene in vivo results in a widespread ectopic expression (right). We did this in parallel for 300 ligands and receptors to functionally test them in one mouse.
We modified our metastasis model (syngeneic colorectal cancer organoids) to express an elegant niche-labeling construct from the Ilaria Malanchi lab which allowed us to sort metastasis-neighboring and distal hepatocytes, and quantify their integrated sgRNAs as a readout of the screen.
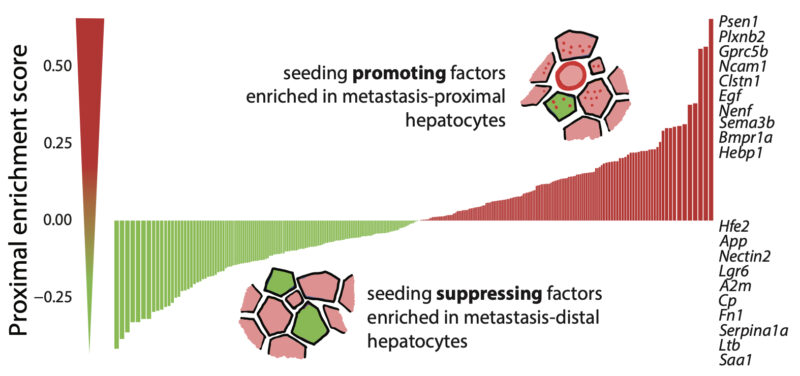
Seeding-promoting factors are enriched next to mets, suppressing ones are depleted. Top hits are predicted to interact with tumor cells in human liver mets (thanks Jonas Kretz and Ati), and with cancer genes more often mutated in mets than in primary tumors (kudos Elena Gonzalez).
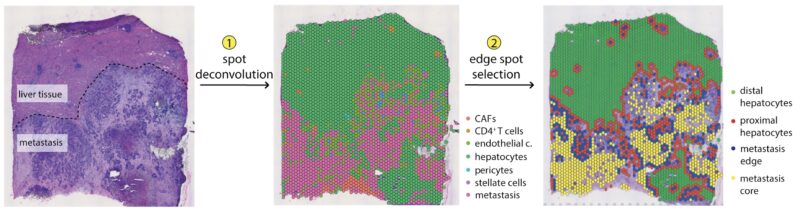
The main hit of our screen is plexin B2. When overexpressed in hepatocytes using AAVs (thanks Morgan Roberts) plexin B2 increased colonization, when knocked out, it prevented mets from intrasplenic injection and orthotopic tumors (thanks to Hassan Fazilaty, Tomas Valenta).
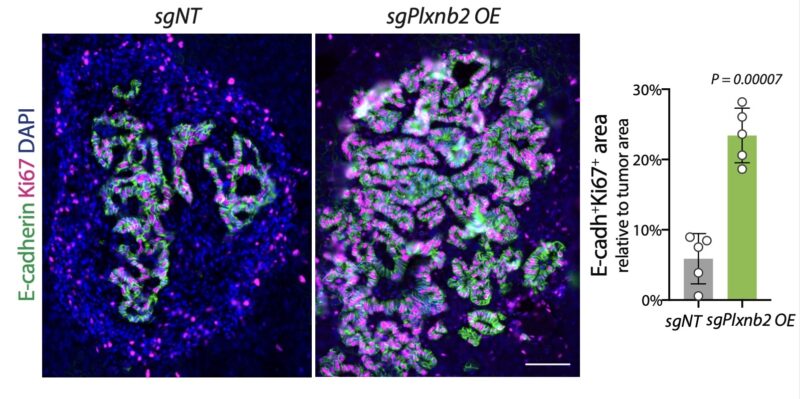
We uncovered that liver-derived plexin B2 promotes the seeding of incoming tumor cells by activating the transcription factor Klf4, and thereby inducing the epithelialization of metastases. In the absence of plexin B2, metastases lack Klf4 expression and retain Zeb1 expression.
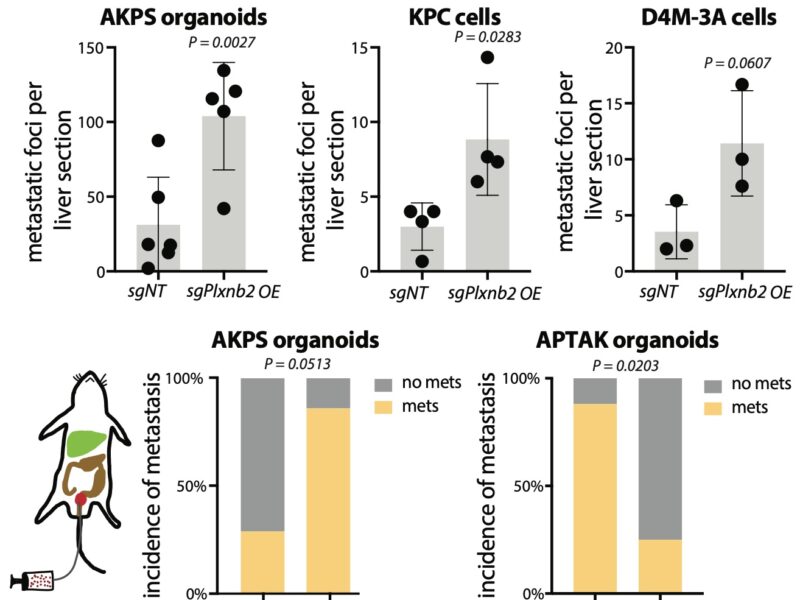
Plexin B2 is postulated to interact with class IV semaphorins; thus we created a quadruple-KO (great work by Ab Meijs and Randall Platt) of these semaphorins in cancer organoids & observed similar phenotypes as in plexin B2 KO livers: decreased metastatic rate & absence of Klf4.
The expression of these semaphorins in human primary CRC is highest in high relapse cells recently identified by the BattleLab, and correlates with a shorter recurrence-free survival, indicating that this interaction module might also play an important role in human patients.
Our findings highlight the essential role of hepatocytes for metastatic seeding and suggest that epithelialization is required for the adaptation of CRC metastases to the liver environment.
Finally, our screening approach, which evaluates host-derived signals rather than tumour-intrinsic factors for their ability to promote metastatic seeding, lays a framework for the screening of environmental constraints to metastasis in other organs and cancer types.
Such work takes a village! I would like to thank Davide Eletto for designing and cloning of all plasmids and vectors, Marie for support and guidance with animal experimentation, Srinivas Narayanan, ETH Zurich, and Krebsliga Schweiz for funding and everybody who contributed ideas, help, and reagents.
And please read the great accompanying perspective.
Thanks so much, Katharina Woess and Direna Alonso-Curbelo!”
Source: Andreas Moor/X


