Breaking News: EMA Joins FDA in Approving Osimerintib with Chemotherapy as a New 1st-Line Treatment for EGFR-Mutated Advanced Lung Cancer
The European Medicines Agency (EMA) has just approved Tagrisso (Osimertinib) in combination with chemotherapy as a groundbreaking first-line treatment for patients with EGFR-mutated advanced lung cancer.
This exciting development, based on the compelling results from the FLAURA2 trial, marks a significant leap forward in lung cancer care, extending median progression-free survival by nearly nine months compared to the standard of care.

In a celebratory social media post, Dr. David Planchard (@dplanchard), Principal Investigator of the trial led to approval shared his enthusiasm: “Great news, a new option in our 1st line strategy for EGFRmut patients! To be discussed especially in our patients with brain metastases, ctDNA+ or high tumor load at diagnosis.”
This approval on Jul 5, 2024 is a game-changer, especially for patients with complex cases involving brain metastases or high tumor load at diagnosis. The oncology community eagerly anticipates the positive impact this new treatment regimen will have on patient outcomes.
The Journey to Osimertinib Plus Chemotherapy Approval
The landscape of non-small cell lung cancer (NSCLC) treatment has undergone a remarkable transformation in recent years, particularly for patients with epidermal growth factor receptor (EGFR) mutations.
Before EMA, on Feb 2024 the FDA approved osimertinib (Tagrisso) in combination with chemotherapy as a first-line treatment for patients with EGFR-mutated advanced non–small cell lung cancer (NSCLC). The later marks a significant milestone in the ongoing evolution of targeted therapies for this subset of lung cancer patients.
This article delves into the journey that led to this groundbreaking approval, exploring the previous standard of care, the pivotal FLAURA2 trial, and the implications for future treatment strategies.
The EGFR-Mutated NSCLC Landscape: Before Osimertinib Plus Chemotherapy
The treatment landscape for EGFR-mutated NSCLC has evolved dramatically over the past two decades. Before the advent of targeted therapies, patients with this type of lung cancer faced limited options, primarily relying on traditional chemotherapy with its associated toxicities and modest efficacy.
First- and Second-Generation EGFR-TKIs
The first-generation EGFR-TKIs, such as erlotinib and gefitinib, revolutionized treatment for patients with EGFR-mutated NSCLC. These drugs demonstrated superior efficacy and tolerability compared to traditional chemotherapy, leading to their widespread adoption as first-line treatments. However, resistance inevitably developed, often due to the emergence of the T790M mutation.
Second-generation EGFR-TKIs like afatinib were developed to overcome some of the limitations of first-generation inhibitors. While they showed improved efficacy against certain EGFR mutations, they still faced challenges with acquired resistance and increased toxicity due to their activity against wild-type EGFR.
The Osimertinib Era
The introduction of Osimertinib, a third-generation EGFR-TKI, marked another significant leap forward. Osimertinib was designed to target both the common EGFR mutations (exon 19 deletions and L858R) and the T790M resistance mutation while sparing wild-type EGFR.
The FLAURA trial demonstrated the superiority of Osimertinib over first-generation EGFR-TKIs in the first-line setting, leading to its approval and establishment as the standard of care for newly diagnosed EGFR-mutated advanced NSCLC.
The FLAURA2 Trial: A Game-Changer
The FLAURA2 trial, which led to this latest approval, was designed to address the ongoing challenge of disease progression despite initial response to EGFR-TKI therapy. This phase 3, international, open-label study enrolled 557 patients across more than 150 centers in over 20 countries.
Design and methods:
Patients were randomly assigned in a 1:1 ratio to receive Osimertinib plus chemotherapy (with pemetrexed and a platinum-based agent) or osimertinib monotherapy.
Key findings from FLAURA 2 Trial
Progression free survival (PFS)- PFS results depend on many criterias- mutation type, CNS metastasis
- Patients with exon 19 deletion, 27.9 months in the Osimertinib–chemotherapy group and 19.4 months in the osimertinib group
- Patients with L858R mutation- 24.7 months and 13.9 months, respectively.
- Patients with CNS metastases at baseline- 24.9 months and 13.8 months, respectively
- Patients without CNS metastases at baseline- 27.6 months and 21.0 months, respectively.
Using Osimertinib together with platinum-based chemotherapy improved median progression-free survival by 38% compared to using Osimertinib alone. The combination showed an 8.8-month increase in progression-free survival based on investigator assessment and a 9.5-month increase based on blinded independent central review.
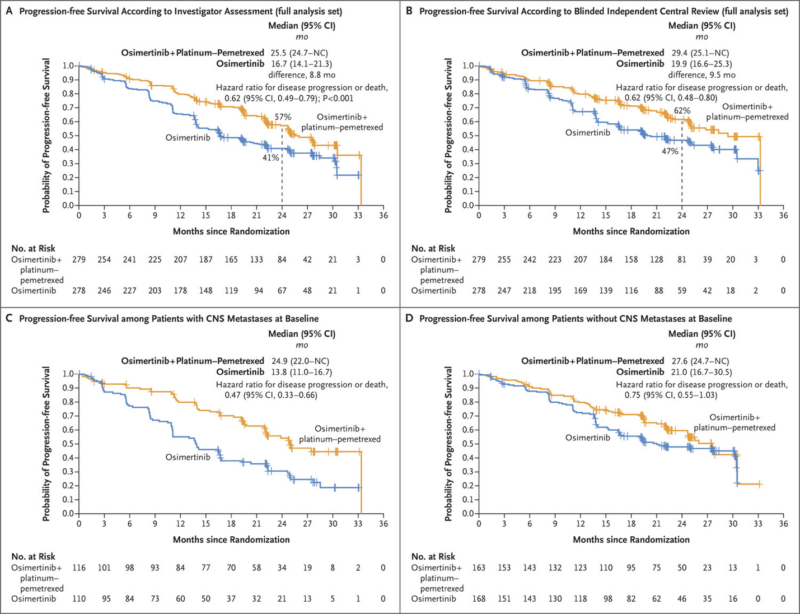
- The hazard ratio for disease progression or death was 0.62 in favor of the osimertinib plus chemotherapy arm.
- At 24 months, 57% of patients in the combination arm were alive and progression-free compared to 41% in the osimertinib monotherapy arm.
- The most common grade ≥3 adverse events were hematologic toxicities, which were higher with the combination but consistent with the known safety profiles of the individual agents.
Implications for Patient Care
The approval of Osimertinib plus chemotherapy offers several potential benefits for patients:
- Extended progression-free survival: The combination therapy provides nearly 9 additional months before disease progression compared to osimertinib alone.
- Improved outcomes for high-risk patients: Patients with brain metastases or more aggressive disease subtypes may particularly benefit from the intensified approach.
- Flexibility in treatment options: Clinicians now have the choice between osimertinib monotherapy and the combination approach, allowing for more personalized treatment strategies.
- Potential for deeper and more durable responses: The combination of targeted therapy and chemotherapy may lead to more profound tumor responses and longer-lasting disease control.
Expert Perspectives
Dave Fredrickson, Executive Vice President of AstraZeneca’s Oncology Business Unit, stated, “This approval reinforces Tagrisso as the backbone therapy in EGFR-mutated lung cancer either as monotherapy or in combination with chemotherapy.
This is especially important for those with more aggressive disease, including patients whose cancer has spread to the brain and those with L858R mutations.”
“This approval brings a critical new treatment option to patients with advanced EGFR-mutated non-small cell lung cancer,” said Pasi A. Jänne, MD, PhD, medical oncologist at Dana-Farber Cancer Institute and principal investigator for the trial.
“Now, with the choice of two highly effective osimertinib-based options, physicians can better tailor treatment to an individual’s needs and help ensure the best possible outcome for each patient,” added Jänne.
Dr. David Planchard, a thoracic oncologist at Gustave Roussy Institute of Oncology and principal investigator for the trial, hailed this as “a significant advance,” and said: “Today’s news marks a significant advance for patients with EGFR-mutated lung cancer in Europe, providing a new 1st-line treatment option with osimertinib now in combination with chemotherapy.
The FLAURA2 results build on the established efficacy of Osimertinib monotherapy, showing a meaningful nine-month improvement in progression-free survival and offering physicians the option to tailor treatment to a patient’s specific needs.”
You can read the full article :
The combination therapy is approved is now approved in more than 110 countries, including the US, EU, China, and Japan. Additional countries will likely approve the combination as regulatory applications are under review in several countries based on the FLAURA2 results.
- European Union (EU): Approved on July 5, 2024
- United States (US): Approved on February 16, 2024
- China: Approved on June 26, 2024
- Japan: Approved (specific date not mentioned)
Key Conferences Where FLAURA2 Results Were Presented:
1. 2023 World Conference on Lung Cancer (September 11, 2023): Primary results of the FLAURA2 trial were presented.
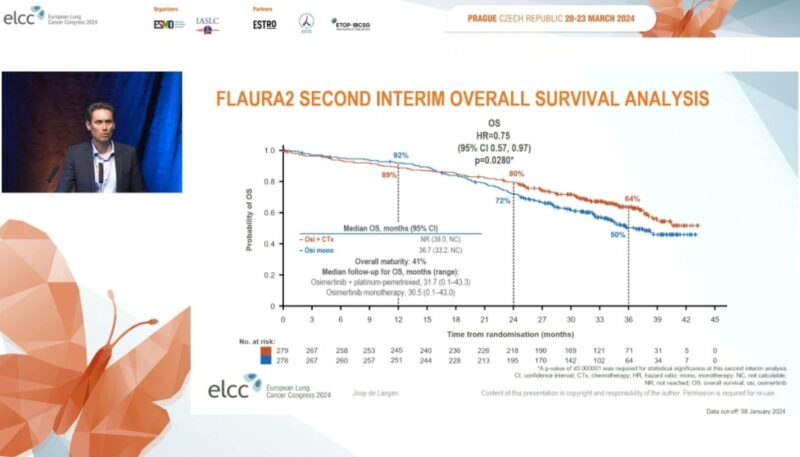
2. 2024 European Lung Cancer Congress: Updated overall survival data were presented, showing a trend towards an OS benefit with Osimertinib plus chemotherapy.
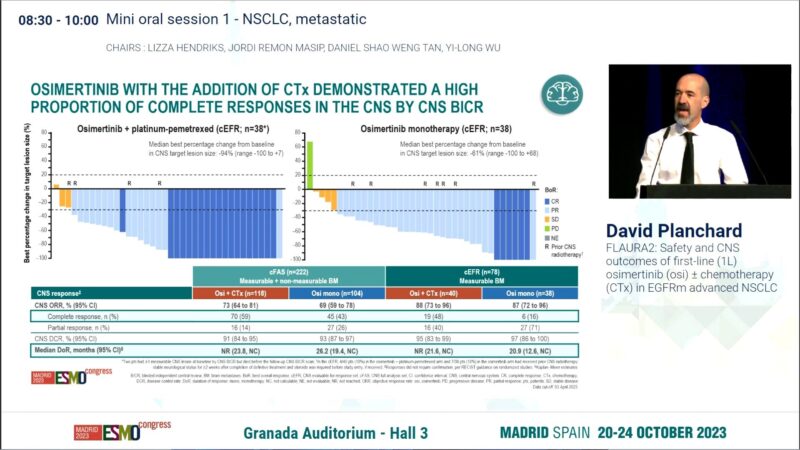
3. ESMO Congress 2023 (October 21, 2023): Safety and CNS outcomes from FLAURA2 were presented, demonstrating a clinically meaningful reduction in the risk of CNS progression for patients with brain metastases.
4. 2023 ESMO Asia Congress: Prespecified exploratory analysis of Chinese patients from FLAURA2 was presented, showing consistent benefits in this population.
5. JLCS 2023 (On-demand) – First-line Osimertinib ± Platinum-Pemetrexed for EGFRm Advanced NSCLC FLAURA2 Japan subset

From @KatsuakiMaehara Twitter post
6. AACR 2024 (Plenary) – FLAURA2: exploratory analysis of baseline (BL) and on-treatment plasma EGFRm dynamics in patients (pts) with EGFRm advanced NSCLC treated with first-line (1L) osimertinib (osi) ± platinum-pemetrexed
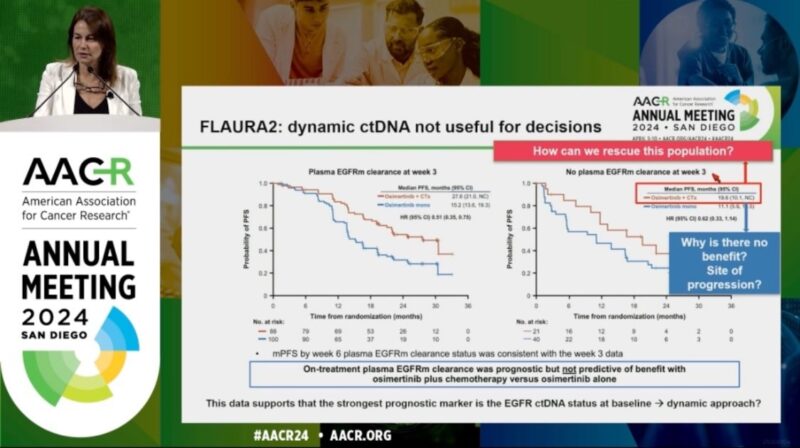
These presentations at major oncology conferences have helped to disseminate the FLAURA2 results to the global oncology community, highlighting the significant improvement in progression-free survival and the manageable safety profile of the combination therapy.
The data from these presentations have supported the regulatory approvals in multiple countries and regions, potentially changing the standard of care for patients with EGFR-mutated advanced NSCLC.
Other key trials with osimertinib
Osimertinib (Tagrisso) has been extensively evaluated in many other clinical trials for the treatment of EGFR-mutated non-small cell lung cancer (NSCLC)
ADAURA Trial
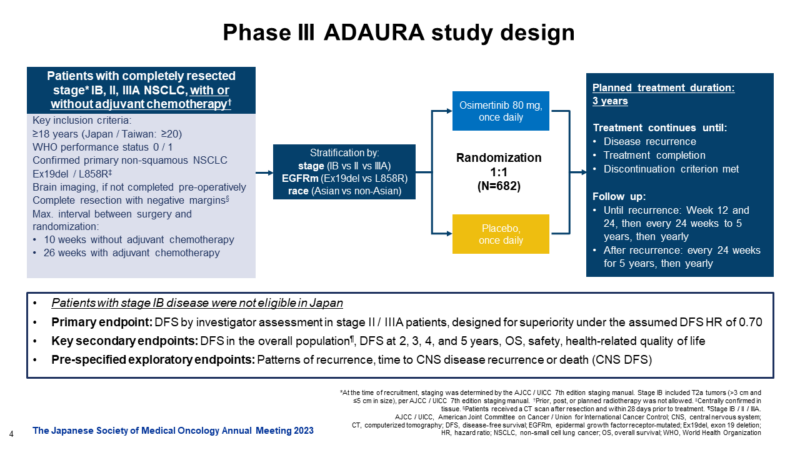
Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC, Masahiro Tsuboi, M.D., Roy S. Herbst, M.D., Ph.D., Thomas John, M.B., B.S., Ph.D., Terufumi Kato, M.D., Margarita Majem, M.D., Ph.D., Christian Grohé, M.D., Jie Wang, M.D., Ph.D., +13, for the ADAURA Investigators* N Engl J Med 2023;389:137-147, DOI: 10.1056/NEJMoa2304594, VOL. 389 NO. 2
- The phase III ADAURA trial evaluated osimertinib as adjuvant therapy after complete tumor resection in patients with early-stage EGFR-mutated NSCLC.
- In patients with stage IB to IIIA EGFR mutation–positive NSCLC, disease-free survival was significantly longer among those who received osimertinib than among those who received placebo. The percentage of patients who were alive and disease-free at 24 months was 89% (95% CI, 85 to 92) in the osimertinib group and 52% (95% CI, 46 to 58) in the placebo group (overall hazard ratio for disease recurrence or death, 0.20; 99.12% CI, 0.14 to 0.30; P<0.001). the median disease-free survival was not reached (95% CI, could not be calculated to could not be calculated) in the osimertinib group and 27.5 months (95% CI, 22.0 to 35.0)
- The trial was led by investigators including Roy S. Herbst, MD, PhD, of Yale Cancer Center.
AURA3 Trial

Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer: NEJM
February 16, 2017, N Engl J Med 2017;376:629-640, DOI: 10.1056/NEJMoa1612674
- The phase III AURA3 trial compared osimertinib to standard chemotherapy in patients with EGFR T790M mutation-positive advanced NSCLC who progressed on prior EGFR-TKI therapy.
- The median duration of progression-free survival was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 months vs. 4.4 months; hazard ratio; 0.30; 95% confidence interval [CI], 0.23 to 0.41; P<0.001). The objective response rate was significantly better with osimertinib (71%; 95% CI, 65 to 76) than with platinum therapy plus pemetrexed (31%; 95% CI, 24 to 40) (odds ratio for objective response, 5.39; 95% CI, 3.47 to 8.48; P<0.001).
- The trial was led by investigators including Suresh S. Ramalingam, MD, of Emory University.
Top ongoing trials with osimertinib
1. LAURA Phase III Trial
- Objective: To evaluate the efficacy of osimertinib in patients with unresectable, Stage III EGFR-mutated NSCLC.
- Status: Currently under review with global regulatory authorities.
- Details: This trial aims to determine whether osimertinib can provide a significant benefit in progression-free survival (PFS) and overall survival (OS) in patients who have not undergone surgery.
2. NeoADAURA Phase III Trial
- Objective: To assess the use of osimertinib in the neoadjuvant setting for patients with resectable EGFR-mutated NSCLC.
- Status: Results expected later this year.
- Details: This trial investigates whether administering osimertinib before surgery can improve surgical outcomes and long-term survival.
3. ADAURA2 Phase III Trial
- Objective: To evaluate osimertinib as an adjuvant treatment in early-stage, resectable EGFR-mutated NSCLC.
- Status: Ongoing.
- Details: Building on the success of the ADAURA trial, ADAURA2 aims to confirm the benefits of osimertinib in preventing recurrence after surgery.
4. SAVANNAH Phase II Trial
- Objective: To explore the combination of osimertinib with savolitinib, a MET inhibitor, in patients who have developed resistance to osimertinib.
- Status: Ongoing.
- Details: This trial targets mechanisms of resistance to osimertinib, aiming to extend its efficacy in patients who no longer respond to monotherapy.
5. ORCHARD Phase II Trial
- Objective: To investigate various combination therapies with osimertinib in patients with advanced EGFR-mutated NSCLC.
- Status: Ongoing.
- Details: This trial is designed to identify the most effective combination strategies to overcome resistance and improve patient outcomes.
6. SAFFRON Phase III Trial
- Objective: To test the combination of osimertinib with savolitinib in a larger patient population.
- Status: Ongoing.
- Details: Following promising results from the SAVANNAH trial, SAFFRON aims to confirm the benefits of this combination in a broader clinical setting.
7. OSIRIS Trial
- Objective: To study osimertinib resistance mechanisms in patients with non-small cell lung carcinoma that have progressed on osimertinib treatment.
- Key Points:
- Aims to analyze resistance mechanisms through both tissue biopsies and circulating tumor DNA.
- Will compare sensitivity and specificity of ctDNA vs. tumor biopsy for detecting resistance mechanisms.
- May help identify new targets for molecular treatment after osimertinib resistance.
- Status: Recruiting, with estimated enrollment of 200 patients.
8.Osimertinib Plus Bevacizumab in Untreated EGFR Exon21 L858R Mutated NSCLC
- Objective: To evaluate the efficacy and safety of osimertinib plus bevacizumab versus osimertinib monotherapy in treatment-naïve advanced NSCLC patients with EGFR exon 21 L858R mutation.
- Key Points:
- Randomized phase 2 study with 90 planned participants.
- Experimental arm: Osimertinib 80 mg daily + Bevacizumab 15 mg/kg every 3 weeks.
- Control arm: Osimertinib 80 mg daily monotherapy.
- Primary endpoint is progression-free survival.
- Status: Not yet recruiting, estimated study completion date May 2025.
Exploring New Frontiers in EGFR-Mutant NSCLC: Future Research Directions
The treatment landscape for patients with non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations has evolved significantly in recent years. The development of highly potent and selective EGFR tyrosine kinase inhibitors (TKIs) has transformed the management of this molecular subtype of lung cancer.
However, as the field continues to advance, researchers are exploring new avenues to further improve outcomes for patients with EGFR-mutant NSCLC.
Optimizing Treatment Schedules and Dosing
One key area of future research will be investigating ways to optimize treatment schedules and dosing of EGFR-targeted therapies. As noted in the search results, “with increasing health care costs, future research should also invest in optimizing treatment and dosing schedules.”
This could involve evaluating alternative dosing regimens or treatment schedules to improve the cost-effectiveness of these targeted agents without compromising efficacy.
Identifying Predictive Biomarkers
Another important direction is the identification of predictive biomarkers that can help select patients most likely to benefit from specific EGFR-directed treatments, including combination approaches.
The search results highlight the need to uncover “clinical or molecular features that correlate with the benefit” of combination strategies, such as the addition of chemotherapy to osimertinib. Leveraging data from clinical trials like FLAURA2 will be crucial in this endeavor.
Overcoming Resistance Mechanisms
A major challenge in the management of EGFR-mutant NSCLC is the development of acquired resistance to EGFR TKIs over time. Future research will likely focus on better understanding the molecular mechanisms driving resistance, which could inform the development of novel, mutant-selective EGFR inhibitors or combination strategies to overcome these resistance pathways.
Expanding to Early-Stage Disease
The search results mention the ADAURA trial, which evaluated the use of osimertinib as adjuvant therapy in early-stage, resectable EGFR-mutant NSCLC. Exploring the role of EGFR-targeted therapies in the curative-intent setting represents an important area of future investigation, with the potential to improve long-term outcomes for these patients.
Combination Strategies with Immunotherapy
The search results also discuss ongoing trials evaluating the combination of osimertinib with the anti-PD-L1 agent durvalumab. Exploring the interplay between EGFR mutations, EGFR-targeted therapies, and immune checkpoint inhibitors could uncover new combination approaches to further enhance the efficacy of treatment for EGFR-mutant NSCLC.
In summary, the future research directions in EGFR-mutant NSCLC are multifaceted, focusing on optimizing treatment regimens, identifying predictive biomarkers, overcoming resistance, expanding to earlier disease stages, and exploring novel combination strategies.
These efforts will be crucial in continuing to improve outcomes and quality of life for patients living with this molecular subtype of lung cancer.
Written by Sona Karamyan, MD


