Kai Rejeski, Visiting investigator at Memorial Sloan Kettering Cancer Center, shared on X:
“Hot off the press
A (perhaps) underestimated aspect of CAR T-cell therapy: non-relapse mortality.
Check out our new meta-analysis on this clinically relevant topic – out today in Nature Medicine!
Let’s take a deeper dive!
First off, what is nonrelapse mortality?
- All deaths not preceded by recurrent or progressive primary malignancy
- It is typically depicted as a cumulative incidence function, treating relapse as a competing risk
- Well described in the field of stem cell transplantation
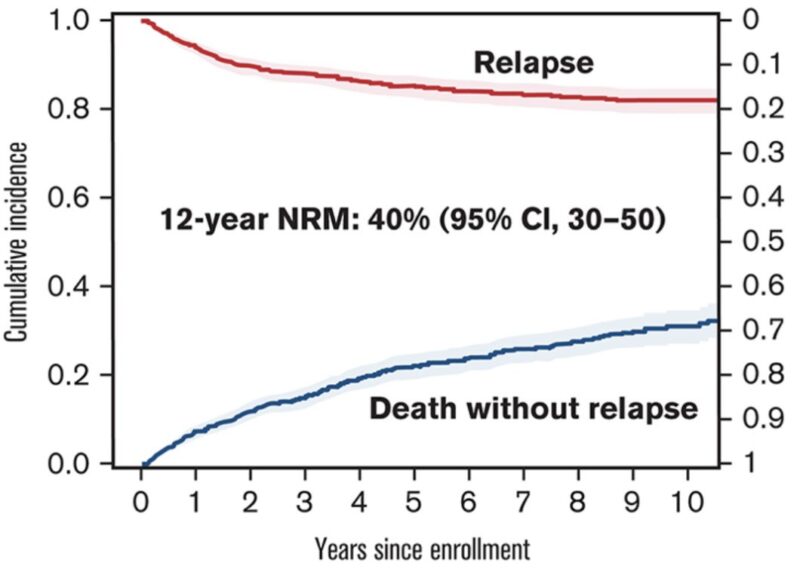
To approach NRM in CAR-T recipients, we performed a systematic review and meta-analysis
Using stringent search and screening criteria, we identified 18 clinical trials and 28 real world studies, spanning 7,604 patients.
PRISMA flow diagram below:
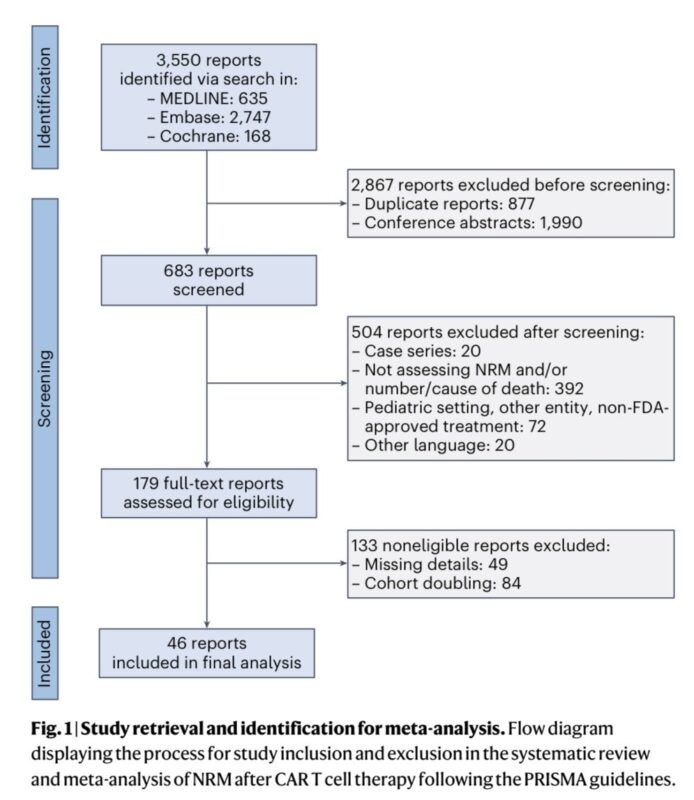
One of the first things to highlight:
- none (!) of the 18 clinical trials reported NRM using the typical cumulative incidence function – highlighting clear deficiencies in NRM reporting
- 14/28 RWs reported NRM
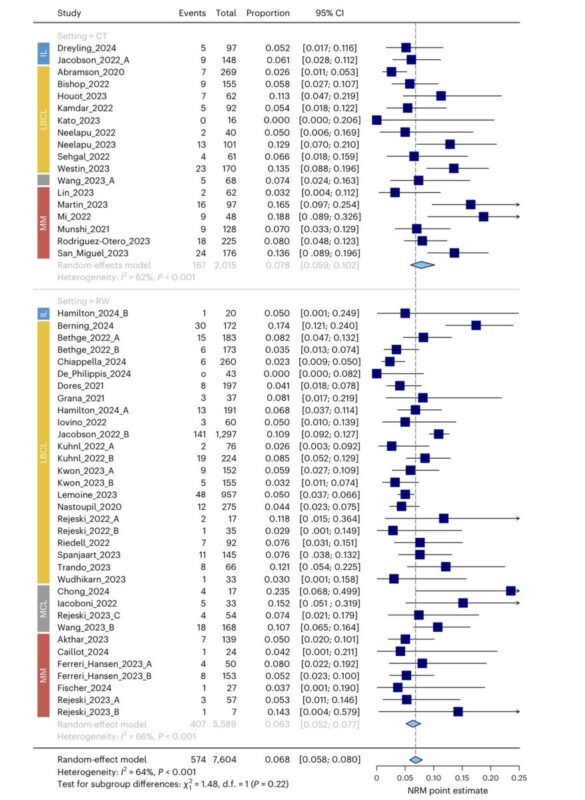
To compensate for the lack of NRM data in CTs and some RWs, we determined NRM point estimates using random-effect models based on proportions.
We show that these derived NRM point estimates were not significantly different than reported NRM rates when both were available.
So, what are the topline results?
With a median follow-up of 13.4 mo, the overall NRM point estimate was 6.8% across all studies.
In simpler words, 1 out of 15 patients receiving CAR-T died of a cause not related to disease progression or recurrence.
More on causes below…
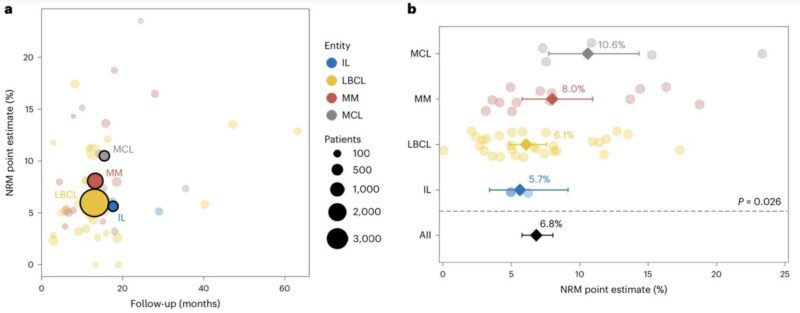
We found considerable heterogeneity in NRM estimates across disease entities:
- Highest in MCL and MM
- Lowest in LBCL and FL
Notably, meta-regression analyses revealed that CAR-T products impact NRM in a disease-specific manner
For LBCL:
- Axi-Cel was associated with significantly higher NRM compared with Liso-Cel and Tisa-Cel (7.4% vs. 3.8% vs. 4.1%; P = 0.004).
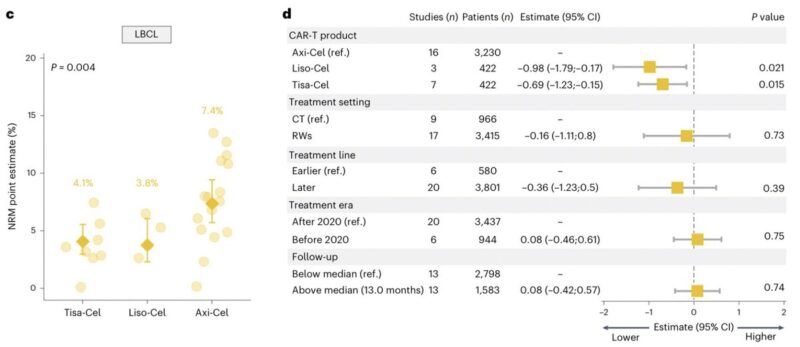
For Multiple Myeloma:
- Cilta-Cel was associated with significantly higher NRM compared with Ide-Cel
(15.2% vs. 6.3%; P < 0.001).
Both of these analyses accounted for key study-level confounders.
What were the main cause of NRM?
Infections, infections, infections…
All in all, they accounted for >50% of attributable NRM events!
A relevant proportion of pts died of Covid 19 infections during the pandemic.
The underlying infection cause was often not attributed.
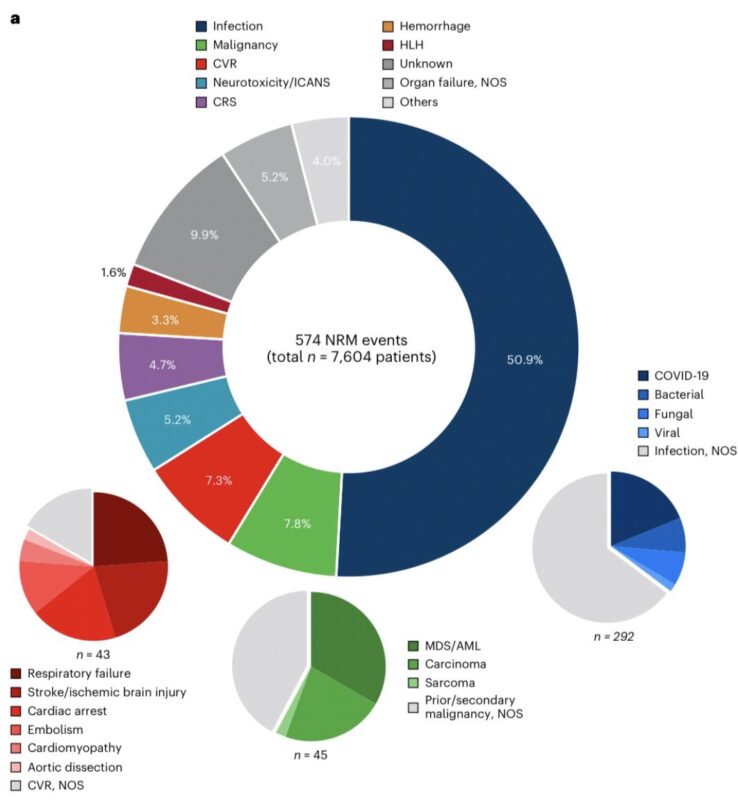
The second and third most common cause were secondary malignancies (7.8% of NRM events) and cardiovascular events (7.3%), respectively.
When we look at the entire cohort, only 0.59% of pts died of a secondary malignancy.
Nonetheless, a relevant contribution to CAR-T NRM.
Notably, the prototypical CAR-T side effects CRS, ICANS and HLH represented only a minority of nonrelapse deaths (cumulatively 11.5%).
However, in most presentations (and papers) these are the side effects that are prominently featured when it comes to featuring CAR-T safety.
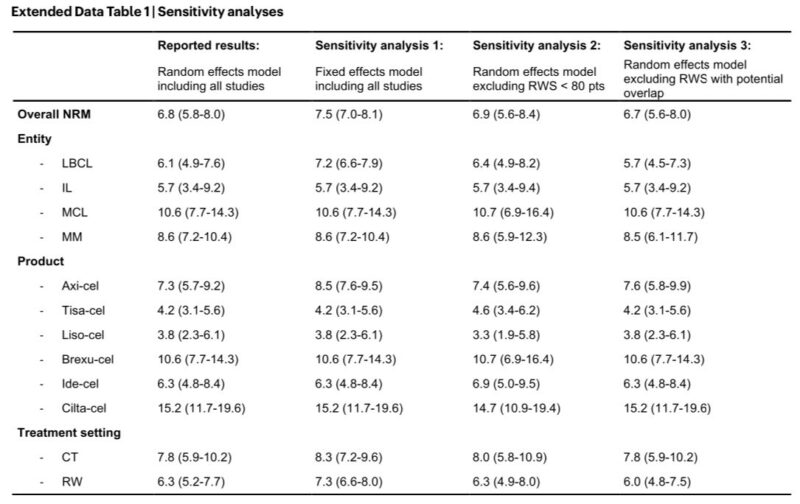
The robustness of the main study findings could be confirmed in several sensitivity analyses:
What are the main conclusions?
- These findings underline the critical importance of infections in patients receiving CD19 and BCMA CAR-T.
Vigilance in reporting, prevention, and management of infections in this vulnerable patient population is paramount - NRM including all specific causes of death should be reported in CAR-T clinical trials.
This will help us evaluate the relative safety of one product vs another using standardized metrics of reporting the safety of cell therapy products. - Product specific differences in NRM emerged for both LBCL and MM.
In the absence of randomized controlled clinical trials, NRM may be one (of many) factors to consider when deciding between one CAR product vs. another.
Others: efficacy, logistics, availability, cost…
I want to thank everyone involved in this effort, particularly the rockstar first-authors David Cordas dos Santos and Tobias Tix!
Also everyone who provided valuable input along the way: Roni Shouval, Eddie Cliff, Sebastian Theurich, Michael von Bergwelt, Mari Subklewe, Miguel Perales.
Also want to highlight that this was a fantastic tri-institutional effort spanning Memorial Sloan Kettering Cancer Center, Dana-Farber Cancer Institute, LMU Munich Hospital Hematology and Oncology, Ludwig Maximilians University, LMU University Hospital Munich.
Great to see such collaborations enriching the project as a whole!”
Source: Kai Rejeski/X


