On December 17, 2025, the FDA issued a regular approval for rucaparib (Rubraca®), marking the formal conversion of its earlier accelerated authorization into full approval for BRCA-mutated metastatic castration-resistant prostate cancer after prior androgen receptor–directed therapy.
The decision applies to tumors with germline or somatic BRCA alterations, requires use of an FDA-approved companion diagnostic, and follows the drug’s initial accelerated approval granted in 2020.
Confirmatory Evidence From TRITON3
The regular approval was supported by results from TRITON3 (NCT02975934), a randomized, open-label, multicenter phase III trial designed to confirm the clinical benefit of rucaparib in mCRPC.
TRITON3 enrolled 405 patients with mCRPC who had progressed on a prior androgen receptor pathway inhibitor (ARPI) and had not received chemotherapy in the castration-resistant setting. Of these, 302 patients had BRCA mutations and 103 had ATM mutations.
Patients were randomized 2:1 to receive:
- Rucaparib
- Physician’s choice of an androgen receptor pathway inhibitor not previously received (enzalutamide or abiraterone acetate) or docetaxel
Randomization was stratified by performance status, presence of liver metastases, and mutation subtype (BRCA1, BRCA2, or ATM). All patients maintained castrate testosterone levels via ongoing androgen deprivation therapy or prior orchiectomy.
What is Rucaparib and how does it work?
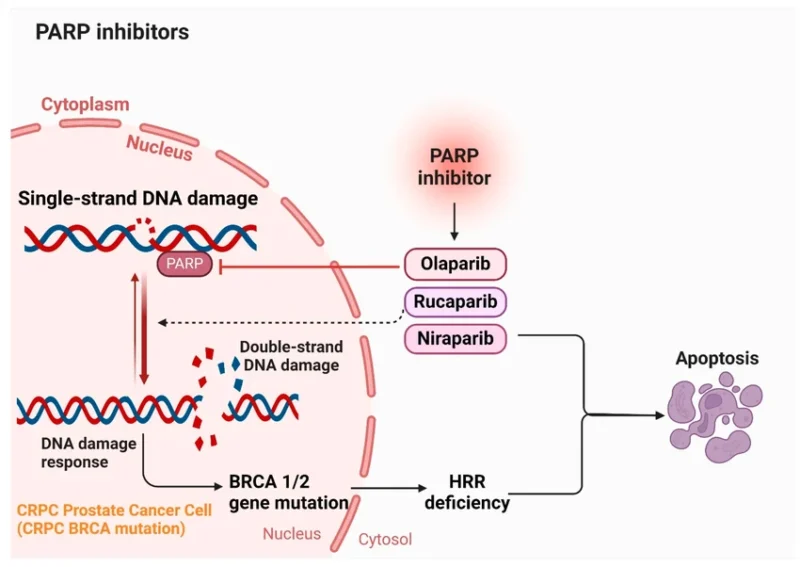
Rucaparib (Rubraca®, pharmaand GmbH), is an oral targeted anticancer therapy belonging to the class of poly(ADP-ribose) polymerase (PARP) inhibitors. PARP enzymes—particularly PARP-1, PARP-2, and PARP-3—play a key role in the repair of single-strand DNA breaks, helping maintain genomic stability during normal cell function.
Rucaparib works by inhibiting PARP enzyme activity, thereby preventing the repair of single-strand DNA damage. During DNA replication, unrepaired single-strand breaks can progress into double-strand breaks. In healthy cells, these lesions are typically resolved through homologous recombination repair, a high-fidelity DNA repair pathway.
In cancer cells with BRCA1 or BRCA2 mutations, homologous recombination repair is defective. When PARP-mediated repair is also blocked by rucaparib, DNA damage accumulates beyond the cell’s capacity to repair it, leading to genomic instability and tumor cell death. This interaction between PARP inhibition and BRCA-associated repair deficiency is known as synthetic lethality.
Through this mechanism, rucaparib preferentially targets tumor cells with underlying defects in DNA damage repair while limiting effects on normal cells with intact repair pathways, providing a biologically rational approach to precision cancer therapy.
source: ResearchGate
Efficacy Outcomes
The primary efficacy endpoint was radiographic progression-free survival (rPFS) assessed by independent central review in patients with BRCA-mutated disease and in the overall study population. Overall survival (OS) was evaluated as a key secondary endpoint.
In patients with BRCA-mutated metastatic castration-resistant prostate cancer (n=302), rucaparib demonstrated a clinically meaningful and statistically significant improvement in rPFS compared with physician’s choice therapy:
- Median rPFS: 11.2 months (95% CI: 9.2–13.8) with rucaparib vs 6.4 months (95% CI: 5.4–8.3) with physician’s choice
- Risk reduction for radiographic progression or death: 50% (HR 0.50; 95% CI: 0.36–0.69; p <0.0001)
- rPFS benefit was also observed in the overall study population
Median overall survival was 23.2 months in the rucaparib arm and 21.2 months in the control arm (HR 0.91; 95% CI: 0.68–1.20), with survival data not statistically significant at the time of analysis. In an exploratory analysis, no meaningful rPFS or OS benefit was observed in patients with ATM mutations, indicating that the overall benefit was primarily driven by outcomes in BRCA-mutated disease.
You can also read about Prostate Cancer Cure Rate: What Patients Should Know in 2025 on OncoDaily.
Safety Profile
The prescribing information for rucaparib includes warnings and precautions for:
- myelodysplastic syndrome / acute myeloid leukemia (MDS/AML)
- Embryo-fetal toxicity
Healthcare professionals should monitor patients for hematologic toxicity and counsel on reproductive risks consistent with PARP inhibitor class effects (FDA, 2025).
Recommended Dosing
The recommended dose of rucaparib is:
- 600 mg orally twice daily
(two 300-mg tablets per dose; total daily dose 1,200 mg)
Treatment is continued until disease progression or unacceptable toxicity, with or without food.
Regulatory Context and Clinical Significance
This application was reviewed using the FDA’s Assessment Aid and was approved one month ahead of the FDA goal date, reflecting an expedited regulatory process.
The conversion of rucaparib’s accelerated approval to regular approval establishes a confirmed standard-of-care option for BRCA-mutated mCRPC, while also clarifying the limited role of PARP inhibition in non-BRCA DNA damage repair subtypes. These findings further underscore the importance of precision genomic testing in advanced prostate cancer treatment selection.
Find full information on FDA.


