Chris De Savi, Chief Scientific Officer, Partner of Curie.Bio, shared a post on LinkedIn:
“Novel FDA Drug Approvals 1H2025
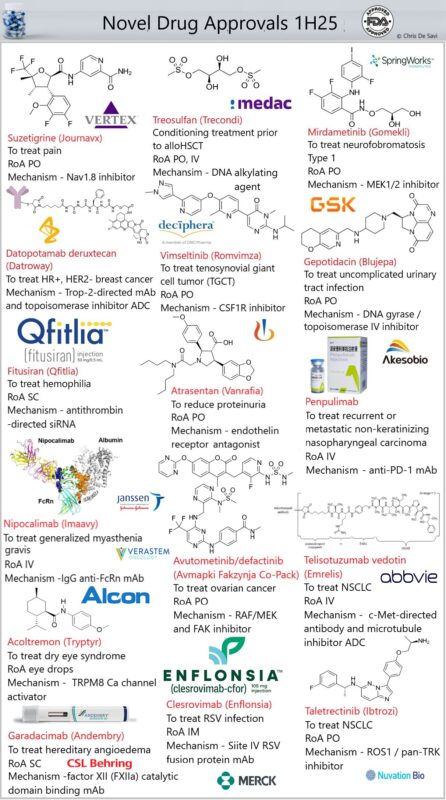
The FDA’s Center for Drug Evaluation and Research (CDER) approved 16 novel drugs in the first half of 2025 — spanning oncology, pain, rare diseases, immunology, and infectious disease prevention.
Here’s the breakdown
- Datopotamab deruxtecan (Datroway) – Trop-2-directed mAb + topoisomerase inhibitor ADC for metastatic HR-positive, HER2-negative breast cancer.
- Treosulfan (Grafapex) – DNA alkylating agent + fludarabine as a preparative regimen for alloHSCT in AML or MDS.
- Suzetrigine (Journavx) – oral Nav1.8 inhibitor, non-opioid analgesic for moderate–severe acute pain.
- Mirdametinib (Gomekli) – oral dual MEK1/2 inhibitor for neurofibromatosis type 1.
- Vimseltinib (Romvimza) – oral CSF1R kinase inhibitor for symptomatic tenosynovial giant cell tumor.
- Gepotidacin (Blujepa) – bacterial DNA gyrase/topoisomerase IV inhibitor for acute cystitis and gonorrhea, incl. MDR strains.
- Fitusiran (Qfitlia) – anti-thrombin siRNA (GalNAc-conjugated) for prophylaxis in hemophilia A or B, with or without inhibitors.
- Atrasentan (Vanrafia) – endothelin A receptor antagonist to reduce proteinuria in primary IgA nephropathy.
- Penpulimab – PD-1 mAb for non-keratinizing nasopharyngeal carcinoma.
- Nipocalimab (Imaavy) – FcRn-binding mAb for generalized myasthenia gravis.
- Avutometinib/defactinib (Avmapki Fakzynja Co-Pack) – for KRAS-mutated recurrent low-grade serous ovarian cancer.
- Telisotuzumab vedotin (Emrelis) – c-Met-directed ADC with microtubule-disrupting payload for NSCLC.
- Acoltremon (Tryptyr) – TRPM8 calcium channel activator for dry eye syndrome.
- Clesrovimab (Enflonsia) – RSV fusion protein-directed mAb for infant passive immunity.
- Taletrectinib (Ibtrozi) – ROS1 / pan-NTRK inhibitor for ROS1-positive NSCLC.
- Garadacimab (Andembry) – factor XII-directed mAb for hereditary angioedema prophylaxis.
My Pick – Nipocalimab
Nipocalimab is a human IgG1 monoclonal antibody that targets the neonatal Fc receptor (FcRn), blocking IgG recycling and lowering harmful autoantibodies without affecting IgG production or triggering immune-mediated cytotoxicity.
Approved for generalized myasthenia gravis, it addresses the disease at its source — reducing the antibodies that impair nerve–muscle communication and cause weakness.
This precision approach may pave the way for treating other antibody-driven conditions.”
More posts featuring Chris De Savi on OncoDaily.


