The FDA has granted orphan drug designation to ZEN-3694, a first-in-class BET bromodomain inhibitor being developed for NUT carcinoma—a rare, aggressive cancer driven by NUTM1 gene fusions and lacking any approved targeted therapies. The designation highlights the urgent unmet need in this disease and reflects early clinical evidence showing that ZEN-3694 can disrupt the epigenetic mechanisms fueling NUT carcinoma while demonstrating encouraging activity, a favorable safety profile, and compatibility with combination regimens in more than 500 patients treated to date.
“Orphan drug status underscores the unmet need for novel treatment options in NUT carcinoma, where patients face poor prognoses and currently have no approved targeted therapies. We believe ZEN-3694, through its epigenetic mechanism and combinatorial approach, has the potential to significantly improve outcomes. Orphan drug designation and the recently announced fast track designation help us advance this program with the goal of making ZEN-3694 available to those who may benefit.”
Said Donald McCaffery, CEO of Zenith Epigenetics
About ZEN-3694
ZEN-3694 targets the key epigenetic process that drives NUT carcinoma. In this disease, NUTM1 fusion oncoproteins—most often BRD4-NUT—abnormally recruit BET proteins and create large “super-enhancer” regions that keep growth-promoting genes constantly active and block normal cell differentiation. This leads to the aggressive, undifferentiated behavior characteristic of NUT carcinoma. ZEN-3694 is a BET bromodomain inhibitor that prevents these BET proteins from binding to chromatin, causing the collapse of the super-enhancers that the fusion protein relies on.
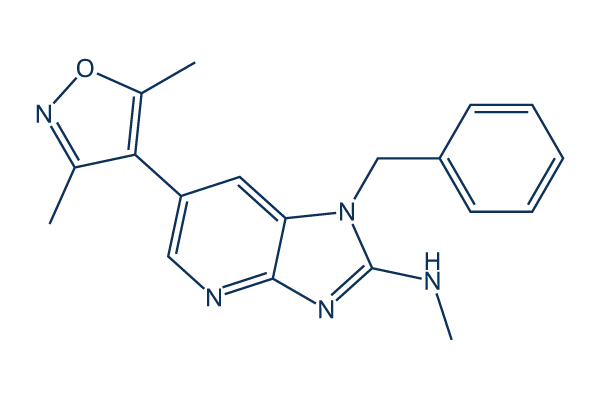
Source: www.selleckchem.com
As these structures break down, the overactive transcription programs are suppressed and cancer cells begin to differentiate and slow their growth. Through this mechanism, ZEN-3694 directly interferes with the core biology of NUT carcinoma and provides a strong rationale for use both alone and in combination with chemotherapy or targeted therapies.
What are NUT carcinomas ?
NUT carcinoma is a rare, highly aggressive epithelial malignancy defined by rearrangements of the NUTM1 gene, most commonly forming BRD4–NUT fusion oncoproteins. It affects patients of all ages but is seen most frequently in young adults, often presenting as a rapidly enlarging mass arising in midline structures such as the sinonasal tract, nasopharynx, or mediastinum, though it can occur in virtually any anatomical site. Histologically, the tumor is composed of primitive, monotonous round to oval cells with high mitotic activity and characteristic foci of abrupt squamous differentiation.
Clinically, NUT carcinoma is associated with a median survival of approximately one year, reflecting its rapid progression, early regional and distant metastasis, and limited responsiveness to conventional chemotherapy or radiation.
Diagnosis requires confirmation of NUTM1 rearrangements—either by molecular testing or by demonstration of the highly specific speckled nuclear positivity on NUT immunohistochemistry. The discovery that NUTM1 fusions block epithelial differentiation and drive uncontrolled proliferation has positioned epigenetic therapies, such as BET bromodomain inhibitors, as a biologically rational strategy under active clinical investigation for this otherwise refractory disease.
Ongoing Studies with ZEN-3694
ZEN-3694 is being evaluated in two ongoing clinical trials focusing on NUT carcinoma, each exploring a different therapeutic strategy.
Phase 1/2 Trial of ZEN-3694 + Cisplatin + Etoposide (NCT05019716)
This international phase 1/2 study is enrolling patients 12 years and older with metastatic or unresectable NUT carcinoma. Diagnosis must be confirmed through WHO-recognized criteria, including ectopic NUT protein expression or detection of NUT gene translocation by sequencing or FISH.
ZEN-3694 is administered orally either once or twice daily on days 1–14 or 1–21 of each treatment cycle. Within the same cycles, cisplatin is given on day 1 and etoposide on days 1–3 for up to four to eight cycles.
The phase 1 study is defining the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) for the triplet regimen. The phase 2 study is assessing overall response rate (ORR) with ZEN-3694 added to standard cisplatin/etoposide chemotherapy.
This design explores whether epigenetic modulation can sensitize NUT carcinoma to established cytotoxic therapy, a rational approach given the transcription-driven biology of the disease.
Phase 1 Trial of ZEN-3694 + Abemaciclib (NCT05372640)
A second study is investigating ZEN-3694 in combination with the CDK4/6 inhibitor abemaciclib in patients with metastatic or unresectable NUT carcinoma, as well as breast cancer and other solid tumors lacking curative treatment options.
Patients receive ZEN-3694 once daily either continuously (days 1–28) or on a 5-days-on/2-days-off schedule, alongside twice-daily abemaciclib in 28-day cycles. A dedicated dose-expansion cohort is focused exclusively on NUT carcinoma.
The primary objective is to determine the MTD and RP2D of the combination. Secondary objectives include preliminary antitumor activity, capturing progression-free survival, overall survival, ORR, time to response, and duration of response.
This trial tests whether dual disruption of transcriptional regulation (via BET inhibition) and cell-cycle progression (via CDK4/6 blockade) can produce synergistic antitumor effects.
What are orphan drugs?
Orphan drugs are pharmaceutical agents designed to treat rare and “orphan” diseases. In the U.S., a disease is considered rare if it affects fewer than 200,000 people and in UE the threshold is fewer than 5 in 10,000 people.
Generally it takes years and many trials in order to have FDA approval for the drug and only after being approved by FDA pharma companies can sell new medications. It is not profitable to generate a new drug for a rare disease, and that’s why many rare diseases were neglected by pharma companies and they were called “ orphan diseases”.
In order to improve the research and new drug development for these diseases, the U.S. The Orphan Drug Act (ODA) was signed in 1983 into law, which provides financial incentives, market exclusivity (7 years in the U.S., 10 years in the EU), and tax credits. The FDA provides ODD to drugs and biologics that demonstrate potential for the diagnosis and/or treatment of rare diseases.
Conclusion
Together, the orphan drug designation and ongoing clinical development of ZEN-3694 reflect growing momentum toward the first targeted therapy for NUT carcinoma, a disease that has long lacked effective treatment options. By directly disrupting the epigenetic machinery that drives NUTM1-fusion tumors and demonstrating encouraging early clinical activity, ZEN-3694 offers a scientifically grounded and urgently needed therapeutic approach for this highly aggressive cancer.
For more information click here.