Vorasidenib (Voranigo) has received approval from the UK Medicines and Healthcare products Regulatory Agency (MHRA) on 16 September 2025 for the treatment of adults and children aged 12 years and older with grade 2 astrocytoma or oligodendroglioma carrying a susceptible IDH1 or IDH2 mutation.
This approval, granted under Project Orbis, an international regulatory collaboration led by the US Food and Drug Administration (FDA), provides faster access to innovative therapies across multiple countries. The decision represents a major milestone in the management of IDH-mutant gliomas, introducing a targeted therapy option for patients who have undergone surgery but do not yet require immediate chemotherapy or radiotherapy.
“Project Orbis opens access to safe and effective new cancer drugs for patients that need them, and we are assured that the appropriate regulatory standards for the approval of this medicine have been met. As with all products, we will keep the safety of this medicine under close review.”
Julian Beach, MHRA Executive Director, Healthcare Quality and Access, said
What drug is Vorasidenib (Voranigo) and how does it work?
Vorasidenib is an oral, brain-penetrant, dual inhibitor of mutant isocitrate dehydrogenase enzymes (IDH1 and IDH2).
In normal cells, IDH1 and IDH2 enzymes convert isocitrate to α-ketoglutarate (α-KG), a key metabolite in cellular energy production. However, mutations in IDH1 or IDH2—commonly seen in astrocytomas and oligodendrogliomas—alter enzyme function. Instead of producing α-KG, the mutated enzymes generate 2-hydroxyglutarate (2-HG), an oncometabolite.
Excess 2-HG accumulates in cells and disrupts epigenetic regulation, blocking normal cell differentiation and promoting tumor growth.
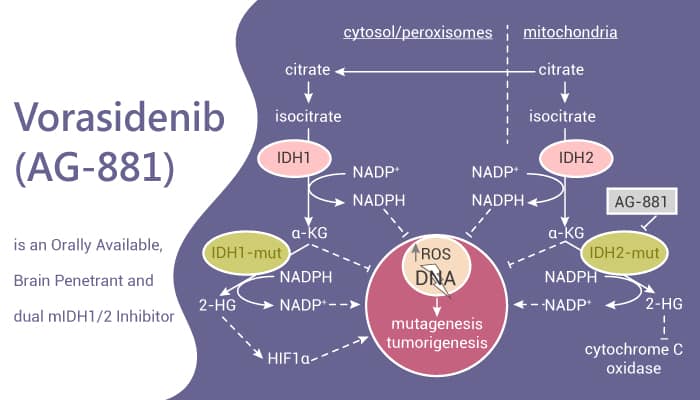
By selectively inhibiting the mutant IDH1/2 enzymes, vorasidenib lowers 2-HG levels, thereby restoring more normal cellular differentiation processes and slowing tumor progression.
Because it crosses the blood–brain barrier, vorasidenib can act directly within the central nervous system, making it especially effective in treating IDH-mutant gliomas.
Understanding the Disease:What are gliomas?
Gliomas are a group of primary brain tumors that arise from glial cells, the supportive cells of the central nervous system. They are classified by the World Health Organization (WHO) into different grades (1–4), with grade 2 gliomas considered “low-grade,” meaning they typically grow more slowly than higher-grade gliomas but are still progressive and ultimately malignant.
Types included in vorasidenib approval
The approval of vorasidenib (Voranigo) applies to two types of WHO grade 2 gliomas:
- Astrocytomas – tumors derived from astrocytes, star-shaped support cells in the brain.
- Oligodendrogliomas – tumors that originate from oligodendrocytes, the cells that produce myelin.
Both tumor types frequently affect young and middle-aged adults, but they can also occur in adolescents.
The role of IDH mutations
A defining feature of many grade 2 gliomas is the presence of mutations in the IDH1 or IDH2 genes. These mutations are seen in approximately 70–80% of low-grade gliomas. They are considered an early event in tumor development and serve as an important molecular marker in modern glioma classification.
IDH mutations drive the accumulation of an abnormal metabolite, 2-hydroxyglutarate (2-HG), which disrupts normal cellular metabolism and epigenetic regulation. This leads to impaired cell differentiation and fuels tumor growth.
Clinical course and unmet need
- Patients often present with seizures, headaches, or neurological deficits depending on tumor location.
- Median age at diagnosis is typically 30–40 years, younger than for most other brain cancers
Management
Standard management involves surgical resection when feasible, followed by careful monitoring.
While radiotherapy and chemotherapy (such as temozolomide or PCV regimen) are effective, they are often delayed in younger patients due to long-term side effects, including cognitive decline, fatigue, and secondary cancers.
This creates a significant unmet need for treatments that can safely delay disease progression and postpone the use of cytotoxic therapies. Vorasidenib directly addresses this need by targeting the molecular driver (IDH mutation) of the disease.
The INDIGO Trial: Evidence Behind Vorasidenib Approval
The INDIGO trial was designed to determine whether vorasidenib could extend progression-free survival (PFS) in patients with IDH-mutant grade 2 gliomas who had undergone surgery as their only prior treatment. Patients were randomized to receive either vorasidenib 40 mg orally once daily or a matching placebo. Key inclusion criteria required histologically confirmed IDH1/2-mutant grade 2 astrocytoma or oligodendroglioma, no prior exposure to chemotherapy or radiotherapy, and measurable non-enhancing disease on MRI.
The primary endpoint was PFS, as assessed by blinded independent review according to modified RANO criteria for low-grade gliomas. Secondary endpoints included time to next intervention (TTNI), objective response rate (ORR), duration of response (DoR), overall survival (OS), tumor growth rate (TGR), and health-related quality of life (FACT-Br).
Study Design
This was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial.
The INDIGO trial enrolled a total of 331 patients who were randomized in a 1:1 ratio to receive either vorasidenib or placebo. Eligible participants had residual or recurrent grade 2 glioma with an IDH1 or IDH2 mutation and had undergone surgery as their only prior treatment. The study was conducted across 84 centers worldwide, including sites in the United States, Europe, Asia, and Canada. Patients were followed for a median of approximately 20 months, providing robust data on progression-free survival and treatment outcomes.
The study population reflected a relatively young cohort, consistent with the epidemiology of low-grade gliomas, with a median age of 40 years. Both astrocytoma and oligodendroglioma subtypes were included, and all patients had a Karnofsky Performance Status ≥80, ensuring adequate baseline functional capacity.
Results
The INDIGO trial met its primary endpoint, demonstrating a significant improvement in PFS for patients treated with vorasidenib.
Median PFS:
- Vorasidenib: 27.7 months
- Placebo: 11.1 months
- Hazard ratio (HR): 0.39 (95% CI, 0.27–0.56; p<0.001)
Time to Next Intervention (TTNI):
- Vorasidenib substantially delayed the initiation of chemotherapy or radiotherapy compared with placebo, reducing the need for early cytotoxic exposure.
Objective Response Rate (ORR):
- Responses were modest but durable, consistent with the slow-growing nature of LGG. Tumor shrinkage was not the primary goal; rather, stabilizing disease and delaying progression was key.
Safety Profile:
- Vorasidenib was generally well tolerated. The most frequent adverse events (≥10%) were increased liver enzymes, diarrhea, abdominal pain, fatigue, and thrombocytopenia. Elevated alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were the most common laboratory findings, necessitating monitoring but rarely leading to treatment discontinuation.
Importantly, treatment with vorasidenib was oral, convenient, and associated with a manageable safety profile, making it a feasible long-term option for patients.
Conclusion
The INDIGO trial provides robust evidence that vorasidenib (Voranigo) improves clinical outcomes in patients with IDH1/2-mutant grade 2 astrocytomas and oligodendrogliomas, offering a new standard of care for those who have undergone surgery but do not require immediate chemotherapy or radiotherapy.
How Treatment Changed After FDA Approval of Vorasidenib
Before the approval of vorasidenib, treatment options for patients with IDH-mutant grade 2 gliomas were limited to surgery, radiotherapy, and chemotherapy. After initial surgical resection, many patients—especially younger ones—were monitored under a “watch and wait” approach until disease progression made radiotherapy or chemotherapy necessary.
While effective, these treatments carry significant long-term risks such as neurocognitive decline, fatigue, infertility, and increased risk of secondary malignancies. This made delaying cytotoxic therapy a key priority in management.
The FDA approval of vorasidenib in August 2024 (based on the Phase 3 INDIGO trial) marked the first time a targeted therapy was available for this patient population. Vorasidenib provided an oral treatment option that directly targets the underlying IDH1/2 mutation, significantly extending progression-free survival (27.7 vs 11.1 months with placebo) and delaying the need for radiotherapy or chemotherapy.
This approval has reshaped treatment algorithms for IDH-mutant grade 2 gliomas. Instead of waiting until symptoms worsen or the tumor progresses to initiate toxic therapy, clinicians can now use vorasidenib as a frontline maintenance therapy after surgery. This approach helps patients maintain quality of life, postpone intensive treatments, and benefit from a therapy tailored to the biology of their tumor.
You can read the full article here.
