Selpercatinib (Retevmo) is a targeted therapy to treat cancers driven by RET gene alterations. This drug is particularly effective in non-small cell lung cancer (NSCLC) and various forms of thyroid cancer. It was first granted accelerated FDA approval in May 2020 for treating metastatic RET fusion-positive NSCLC, advanced or metastatic RET-mutant medullary thyroid cancer (MTC), and radioactive iodine-refractory RET fusion-positive thyroid cancer. Further approvals have expanded its use, making it a crucial treatment option for patients with RET-driven cancers.
What Is Selpercatinib, and How Does It Work?
Selpercatinib is a RET kinase inhibitor targeting abnormal RET proteins in cancer cells. Normally, the RET gene helps regulate cell growth and development. However, in some cancers, RET mutations or fusions lead to uncontrolled tumor growth. Selpercatinib binds to the ATP-binding site of the RET protein, blocking its activity and preventing cancer cells from multiplying. Because of its high selectivity for RET, it is more effective and has fewer side effects compared to older, less specific kinase inhibitors. Importantly, Selpercatinib can cross the blood-brain barrier, making it useful for patients with brain metastases.
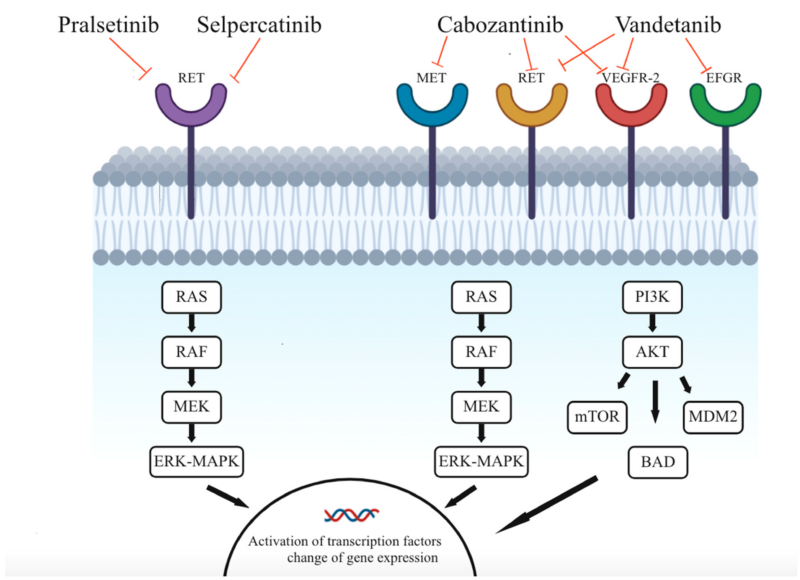
Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int. J. Mol. Sci. 2018, 19, 3258
What Cancers Does Selpercatinib Treat?
Selpercatinib is approved for several RET-driven cancers:
- Non-Small Cell Lung Cancer (NSCLC): Approved for patients with metastatic RET fusion-positive NSCLC.
- Medullary Thyroid Cancer (MTC): Approved for patients with advanced or metastatic RET-mutant MTC requiring systemic therapy.
- Thyroid Cancer: Approved for RET fusion-positive thyroid cancer that is radioactive iodine-refractory and requires systemic therapy.
- Other RET Fusion-Positive Solid Tumors: In September 2022, the FDA granted accelerated approval for patients with locally advanced or metastatic solid tumors harboring RET gene fusions who have no satisfactory alternative treatments.
- Pediatric Patients: In May 2024, the FDA expanded accelerated approval for children aged 2 and older. By September 2024, full FDA approval was granted for medullary thyroid cancer in patients aged 2 and older with specific RET mutations.
Read about Immunotherapy for Lung Cancer on OncoDaily.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Retevmo was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
The effectiveness of Retevmo was carefully studied in a major clinical trial called LIBRETTO-001. This trial looked at how well the drug worked in patients with different cancers caused by changes in the RET gene, including lung and thyroid cancers. Here’s what the results showed:
🫁 Non-Small Cell Lung Cancer (NSCLC)
For patients with RET fusion-positive lung cancer:
- In people who had already received other treatments, about 64% responded well to selpercatinib, meaning their tumors shrank. On average, the response lasted about 17.5 months.
- For those who hadn’t been treated before, the results were even better—85% of patients saw their cancer shrink with selpercatinib.
🦠 Medullary Thyroid Cancer (MTC)
Among patients with RET-mutant MTC:
- 69% of previously treated patients responded to selpercatinib, and most stayed progression-free for at least a year (about 82%).
- In people who hadn’t received any prior treatment, 73% responded, with a 92% chance of remaining progression-free for a year.
🧠 Brain Metastases (CNS Involvement)
One impressive finding was Selpercatinib’s ability to work in the brain.
- In patients whose cancer had spread to the brain, 91% had a positive response—showing that the drug can cross the blood-brain barrier and help control tumors in the central nervous system.
What Other Trials Are Ongoing?
Retevmo has already shown great promise in treating certain cancers, but researchers are still studying it in different ways to improve treatment options and help even more patients. Here are two key areas of ongoing research:
New Study in Children and Teens (Trial NCT06458036)
A current clinical trial is exploring how selpercatinib works in children, teenagers, and young adults who have a type of thyroid cancer called differentiated thyroid cancer with a RET gene fusion.
In this study, patients first receive selpercatinib and then radioactive iodine (RAI) therapy. Researchers want to see if giving selpercatinib first can make the cancer more sensitive to RAI, helping the treatment work better.
Combining Selpercatinib with Other Drugs
Another important area of research is combining Retevmo with other medications, especially in lung cancer (NSCLC).
Sometimes, cancer cells find ways to resist treatment. One known resistance mechanism involves a gene called MET. Researchers are testing whether combining selpercatinib with MET inhibitors (like crizotinib or capmatinib) can overcome this resistance and make treatment more effective.
What Can You Expect During Treatment?
Selpercatinib is a targeted therapy designed to slow cancer growth by blocking abnormal RET proteins. Understanding how it is taken and what to expect during treatment can help patients feel more prepared.
How to Take Selpercatinib?
Retevmo is taken orally, twice a day, as prescribed by your doctor. It can be taken with or without food, but if you are also using proton pump inhibitors (PPIs) for acid reflux or stomach issues, you should take Selpercatinib with food. The capsules or tablets should be swallowed whole, not crushed or chewed. If you miss a dose and it’s more than six hours until your next one, take it as soon as possible. However, if you vomit after taking a dose, do not take an extra pill—just continue with your next scheduled dose.
Monitoring During Treatment
While taking Selpercatinib, regular checkups are necessary to ensure your body is responding well and to monitor for side effects. Your doctor will perform blood tests to check liver function, measure blood pressure, and monitor heart rhythm. These tests help detect any potential issues early, allowing adjustments to be made if needed.
How Long Does Treatment Last?
The length of treatment varies from person to person. Patients typically continue taking Selpercatinib as long as it is controlling their cancer and side effects remain manageable. If the cancer progresses or severe side effects occur, your doctor may adjust the dose or stop the medication.

Learn more about Thyroid Cancer on OncoDaily.
Selpercatinib Side Effects and Management
When taking any medication, it’s natural to be concerned about possible side effects. While most people tolerate treatment well, some may experience certain reactions. The good news is that doctors closely monitor these effects and have ways to help manage them.
Common Side effects
One common side effect is high blood pressure (hypertension). Regular blood pressure checks can help detect any changes early, and if needed, your doctor may prescribe medication to keep it under control.
Changes in liver function can also occur, which is why routine blood tests are important. If any liver enzymes become too high, your doctor may adjust your treatment.
Some people may experience diarrhea or fatigue. Staying well-hydrated, getting plenty of rest, and using supportive medications can make a big difference in managing these symptoms.
Another possible side effect is protein in the urine (proteinuria) or changes in electrolytes, which can affect kidney function. Regular blood and urine tests help catch these issues early so your doctor can take action if needed.
For those with heart conditions, QT prolongation, a change in heart rhythm, may be a concern. If necessary, your doctor will monitor your heart with an ECG to ensure everything stays safe.
Serious side effects
While serious side effects like severe liver problems or allergic reactions are rare, they can sometimes require stopping the medication. If you notice any unusual symptoms—such as yellowing of the skin, severe itching, swelling, or trouble breathing—seek medical attention right away.
By staying in close contact with your healthcare team and keeping up with regular checkups, you can manage these side effects effectively and continue your treatment safely. Always reach out to your doctor if you have any concerns!

What to Expect Long-Term?
Selpercatinib is not a cure but can significantly prolong survival and improve quality of life. The LIBRETTO-001 study showed that treatment-naive patients had a median progression-free survival (PFS) of 22.0 months, while previously treated patients had a PFS of 24.9 months. Long-term monitoring ensures continued effectiveness and safety.
What Should You Avoid During Treatment?
While you’re being treated with selpercatinib (Retevmo), there are a few things you should try to avoid to help the medicine work safely and effectively:
🍊 Grapefruit and Grapefruit Juice
It’s best to skip grapefruit and its juice during treatment. These can interfere with how selpercatinib is broken down in your body, causing higher drug levels than intended. This might increase the risk of serious side effects.
💊 Certain Medications (Strong CYP3A Inhibitors)
Some medicines—like specific antibiotics or antifungal drugs—can affect how your body processes selpercatinib. This can either raise the amount of the drug in your system or lower its effectiveness. If you need one of these medications, your doctor may need to adjust your selpercatinib dose.
❤️ Drugs That Affect the Heart’s Rhythm (QT Prolonging Medications)
Avoid taking medications that can change your heart’s rhythm unless your doctor says it’s absolutely necessary. Selpercatinib can also affect heart rhythm, and combining it with similar drugs could increase the risk of heart problems like irregular heartbeat.
💉 Live Vaccines and Alcohol
During treatment, avoid live vaccines, as your immune system may not be strong enough to handle them safely. It’s also a good idea to limit or avoid alcohol, as it can put extra stress on your liver—which selpercatinib already affects—and increase the risk of side effects.
Real-Life Effectiveness
Beyond clinical trials, real-world data support selpercatinib’s effectiveness. A 2024 case study highlighted a patient who developed MET amplification as a resistance mechanism but regained tumor response after adding a MET inhibitor. Another case in 2023 showed the successful management of resistance using selpercatinib plus capmatinib.
Looking Ahead – The Future of Treatment
Research is ongoing to expand selpercatinib’s indications, improve resistance management, and explore combination therapies. Future developments may refine treatment strategies, making selpercatinib even more effective for patients with RET-driven cancers.
Selpercatinib represents a major advancement in precision oncology, offering hope for patients with RET-altered cancers. As research continues, its role in cancer treatment is expected to evolve further, improving outcomes and expanding treatment options for more patients.
If you are a health care provider, here is the professional version.

You can read about Sofia Vergara and Thyroid Cancer: How She Went Against, How She Survived on OncoDaily.


