Penpulimab is a novel humanized monoclonal antibody that targets PD-1 (programmed death-1), a key immune checkpoint protein. In April 2025, the U.S. Food and Drug Administration (FDA) approved penpulimab-kcqx for two indications in non-keratinizing nasopharyngeal carcinoma (NPC). Penpulimab received approval from the National Medical Products Administration (NMPA) in China for first-line treatment of recurrent or metastatic nasopharyngeal cancer (NPC) combined with chemotherapy. This marks its fourth approved indication, expanding its use across all stages of NPC and reinforcing its role in comprehensive cancer care.
Which company produced Penpulimab?
Penpulimab is jointly developed by Akeso, Inc.. and Sino Biopharm’s Chia Tai Tianqing Pharmaceutical Group. Founded in 2012, Akeso focuses on discovering and commercializing novel therapies for cancer, autoimmune, and inflammatory diseases.
The company has built a strong pipeline of over 50 drug candidates and utilizes cutting-edge platforms like its proprietary Tetrabody bispecific antibody technology. Akeso is also recognized for its end-to-end development capabilities, including in-house manufacturing. In addition to Penpulimab, Akeso has brought several first-in-class and best-in-class immunotherapies to market, positioning itself as a rising leader in global oncology drug development.
How does Penpulimab work?
Penpulimab works by targeting and blocking PD-1 (programmed death-1), a protein found on the surface of T cells (a type of immune cell). Under normal conditions, PD-1 acts as a “brake” on the immune system—it binds to PD-L1 or PD-L2 proteins on other cells to prevent T cells from attacking them. Many cancer cells exploit this pathway by expressing PD-L1, effectively “hiding” from immune detection.
Penpulimab, a humanized anti–PD-1 monoclonal antibody, prevents PD-1 from binding to its ligands (PD-L1 and PD-L2). This releases the brake on the immune system, allowing T cells to recognize and destroy cancer cells more effectively.
What makes Penpulimab unique is its IgG1 subtype with Fc-engineering that minimizes immune-related side effects. Traditional PD-1 antibodies are IgG4, but Penpulimab’s Fc modifications eliminate unwanted immune activation, reducing the risk of inflammatory complications while maintaining strong anti-tumor activity.

Photo from akesobio.com
Penpulimab Approvals: A Comprehensive Timeline of Indications
As of April 2025, Penpulimab has received FDA approval for two key indications in the treatment of non-keratinizing nasopharyngeal carcinoma (NPC):
- First-Line Treatment: Penpulimab is approved in combination with cisplatin or carboplatin plus gemcitabine for adults with recurrent or metastatic non-keratinizing NPC, offering a new frontline immunotherapy-based option.
- Second-Line Monotherapy: It is also approved as a standalone treatment for adults with metastatic non-keratinizing NPC who have progressed following platinum-based chemotherapy and at least one additional line of therapy.
These FDA approvals mark a significant advancement in NPC treatment, positioning Penpulimab as a promising immune checkpoint inhibitor for both newly diagnosed and previously treated patients.
It has received multiple approvals in China from the National Medical Products Administration (NMPA) for various cancer treatments:
- Classic Hodgkin’s Lymphoma (cHL): In August 2021, Penpulimab was approved for patients with relapsed or refractory cHL after at least two lines of systemic chemotherapy. This approval was based on a pivotal trial demonstrating an objective response rate (ORR) of 89.4% and a complete response rate of 47.1% .
- Squamous Non-Small Cell Lung Cancer (sq-NSCLC): In January 2023, Penpulimab, combined with chemotherapy, was approved as a first-line treatment for locally advanced or metastatic sq-NSCLC. The approval was supported by a Phase III trial showing a median progression-free survival of 7.6 months and an ORR of 71.4% .
- Nasopharyngeal Carcinoma (NPC): In March 2025, Penpulimab received approval for first-line treatment of recurrent or metastatic NPC in combination with chemotherapy. Previously, it was approved for third-line treatment of advanced NPC .
These approvals highlight Penpulimab’s expanding role in cancer therapy within China.

You can read more about Nasopharyngeal Cancer: What patients should know on OncoDaily.
What research is behind the approval?
The FDA approval of Penpulimab for first-line treatment of non-keratinizing nasopharyngeal carcinoma (NPC) is based on results from the Phase III AK105-304 trial (NCT04974398)—a randomized, double-blind, multi-center study involving 291 patients with recurrent or metastatic NPC who had not previously received systemic chemotherapy for advanced disease.
Patients were randomized 1:1 to receive:
- Penpulimab + cisplatin/carboplatin + gemcitabine, followed by maintenance Penpulimab
vs. - Placebo + cisplatin/carboplatin + gemcitabine, followed by placebo.
Key Efficacy Results
Primary Endpoint – Progression-Free Survival (PFS):
- Penpulimab group: 9.6 months (95% CI: 7.1–12.5)
- Placebo group: 7.0 months (95% CI: 6.9–7.3)
- Hazard ratio (HR): 0.45 (95% CI: 0.33–0.62), p < 0.0001
12-month PFS rates
- 31% in the Penpulimab group
- 11% in the placebo group
Overall Survival (OS): Still maturing, with no signs of a detrimental trend.
These results demonstrate that Penpulimab significantly extends PFS when added to platinum-based chemotherapy in NPC, offering patients a more effective frontline treatment option.
Subsequent-Line Monotherapy – Phase II AK105-202 Trial
This open-label, multicenter, single-arm Phase II study (NCT03866967) assessed Penpulimab monotherapy in 125 patients with unresectable or metastatic non-keratinizing NPC who had progressed after platinum-based chemotherapy and at least one additional systemic treatment.
Key Efficacy Results
- Objective Response Rate (ORR): 28% (95% CI: 20–37)
- Median Duration of Response (DOR): Not reached (95% CI: 9.2 months to not estimable)
These results demonstrated meaningful and durable antitumor activity in a heavily pretreated population.
Safety Overview
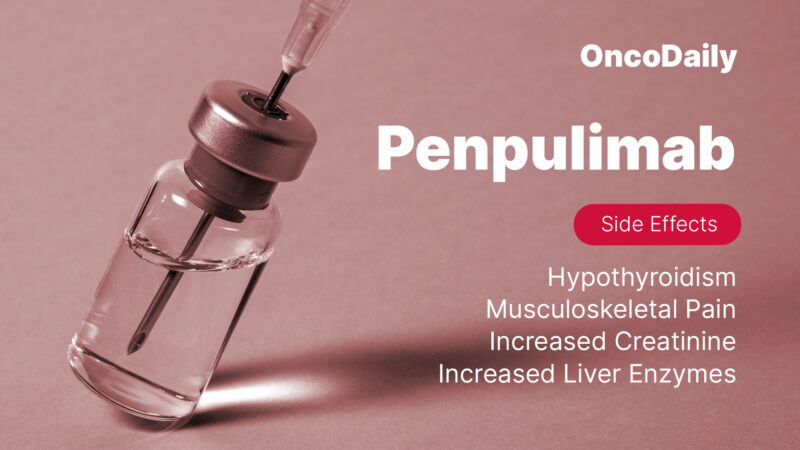
Combination Therapy Adverse Reactions (≥20%): Nausea, vomiting, hypothyroidism, constipation, decreased appetite and weight, cough, COVID-19 infection, fatigue, rash, and pyrexia.
- Monotherapy Adverse Reactions (≥20%): Hypothyroidism and musculoskeletal pain.
- Immune-Mediated Adverse Events: Pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin reactions.
- Fatal Adverse Events (1%): Including pneumonitis, septic shock, colitis, and hepatitis.
These pivotal trials formed the basis for Penpulimab dual FDA approvals, reinforcing its role as a valuable PD-1 inhibitor for both frontline and refractory NPC treatment strategies.
Penpulimab side effects and its management
While it offers therapeutic benefits, it can also lead to various side effects, ranging from common to rare. Understanding these adverse reactions and their management is crucial for optimal patient care.
Common Side Effects
Patients taking this medication may experience changes in their blood work, such as higher levels of creatinine or liver enzymes, and lower levels of phosphate, sodium, albumin, lymphocytes, or hemoglobin. Some people also develop thyroid problems, particularly hypothyroidism, which may be caused by the immune system. Changes in metabolism can occur too, including increased blood sugar and triglyceride levels. Common physical symptoms include muscle or joint pain, fever, respiratory infections, cough, and skin rashes.
Most of these side effects are manageable and can often be controlled with supportive care or adjustments to the treatment dose.
Less Common and Serious Side Effects
In rare cases, treatment may lead to serious immune-related side effects, such as inflammation of the lungs (pneumonitis), bowel (colitis), liver (hepatitis), or hormonal glands, including overactive thyroid function. Severe skin reactions have also been reported, including conditions like Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reactions with widespread rashes and systemic symptoms. Some neurological problems may occur, including muscle weakness, nerve inflammation, or brain swelling. Other serious complications can include heart inflammation (myocarditis), kidney inflammation (nephritis), and strong reactions during or after infusions.
Management of Side Effects
Managing side effects from Penpulimab involves a proactive and structured approach to ensure patient safety and optimize treatment outcomes. Regular monitoring is essential, including routine assessment of liver and kidney function, thyroid hormone levels, and complete blood counts to detect early signs of toxicity. For immune-related adverse events, systemic corticosteroids such as prednisone (1–2 mg/kg/day) are typically initiated, followed by a slow taper over a period of at least one month to prevent recurrence.
Depending on the severity of the reaction, It may need to be temporarily held or permanently discontinued. Supportive care plays a key role as well—for instance, hormone replacement may be required for patients with treatment-induced endocrine disorders, and infusion-related reactions should be managed symptomatically.
Timely recognition and intervention are critical, as early management of side effects not only improves patient comfort but also allows many individuals to safely continue receiving this important immunotherapy.
What is the Recommended Dosage of Penpulimab?
Penpulimab is available as a 100 mg/10 mL single-dose intravenous solution. The dosing depends on the indication:
As monotherapy, for adults with metastatic non-keratinizing nasopharyngeal carcinoma (NPC) who progressed after platinum-based chemotherapy and at least one other prior therapy, the recommended dose is 200 mg IV every 2 weeks, continued until disease progression, unacceptable toxicity, or up to 24 months.
In combination therapy, it used with gemcitabine and either cisplatin or carboplatin as a first-line treatment for recurrent or metastatic NPC. The dose is 200 mg IV every 3 weeks, alongside standard dosing of gemcitabine and platinum agents for six cycles, and Penpulimab is continued for up to 24 months or until progression or toxicity.
Penpulimab Dose Adjustments and Toxicity Management
Treatment may be interrupted or permanently discontinued based on severity and type of side effect. For most immune-related adverse events, therapy is held until improvement to Grade 1 or lower, often with the use of corticosteroids. Permanent discontinuation is recommended for severe or life-threatening toxicities such as Grade 4 pneumonitis, colitis, myocarditis, certain liver toxicities, severe infusion reactions, or confirmed dermatologic syndromes like Stevens-Johnson Syndrome (SJS) or TEN.
Patients with mild to moderate kidney or liver impairment typically do not require dose changes, though severe impairment has not been adequately studied. Overall, ongoing monitoring and timely steroid intervention are key in managing immune-related side effects and maintaining treatment continuity.

Learn about Radiotherapy for Head And Neck Cancer: Types, Success Rate, Side Effects on OncoDaily.
How is Penpulimab administered?
Penpulimab should be diluted with 0.9% NaCl. The solution must be clear to slightly opalescent and colorless or yellowish. If the solution is cloudy, discolored, or contains particles, do not use it.
For preparation, withdraw 20 mL from two 100 mg/10 mL vials (for a 200 mg dose) and dilute in a bag with ≤100 mL of 0.9% NaCl to achieve a final concentration of 2-5 mg/mL. Gently invert the bag to mix; avoid shaking. Discard any unused solution. Administer the diluted solution immediately, or within 4 hours of preparation.
For IV administration, infuse over 60 minutes using a sterile 0.2-0.22 micron filter. In combination therapy, administer Penpulimab first, followed by gemcitabine, and cisplatin/carboplatin last. Do not mix other medications in the same line.
Store unopened vials in the refrigerator at 2-8°C (36-46°F), protected from light. Do not freeze or shake. The diluted solution can be stored at room temperature (20-25°C) or refrigerated, but must be used within 4 hours and should not be frozen.
What to Avoid During Penpulimab Treatment?
During Penpulimab treatment, patients should avoid potential drug interactions, particularly with antibiotics like ceftobiprole and ceftazidime, as these may reduce the drug’s effectiveness. They should also be mindful of immune-mediated side effects, which can affect various organs, and seek medical attention immediately if severe reactions occur. Regular monitoring of liver, kidney, and thyroid functions is advised to detect any adverse effects early. It is essential for patients to consult their healthcare provider before starting or stopping any medication to ensure safety throughout the treatment.
Penpulimab’s effectiveness over time
Penpulimab is a next-generation PD-1 inhibitor under investigation for several cancers, including non-small cell lung cancer, liver cancer, esophageal and head and neck cancers, and melanoma. Its Fc-null IgG1 design reduces immune-related side effects while maintaining strong PD-1/PD-L1 blockade. Already approved for non-keratinizing nasopharyngeal carcinoma (NPC), Penpulimab has shown promising results—improving progression-free survival in first-line treatment and achieving a 28% response rate as monotherapy in previously treated metastatic NPC. It’s also a key part of Akeso’s immunotherapy platform, often used with bispecific antibodies to enhance cancer treatment outcomes.

Learn about Brachytherapy for Head And Neck Cancer: Types, Success Rate, Side Effects on OncoDaily.
Ongoing trials with Penpulimab
Cohort C of this open-label Phase Ib/II study (NCT06821503) investigates LM-108 in combination with penpulimab, albumin-bound paclitaxel, and gemcitabine in patients with advanced solid tumors. Phase Ib established the recommended dose, while Phase II focuses on evaluating the safety and efficacy of this regimen in advanced pancreatic cancer.
The randomized, multicenter trial (NCT06586242) explores the perioperative use of penpulimab plus anlotinib and chemotherapy in patients with locally advanced, resectable esophageal cancer. One group receives oxaliplatin, while the other receives lobaplatin alongside penpulimab, anlotinib, and albumin-bound paclitaxel.
Written by Mariam Khachatryan, MD
FAQ
What is Penpulimab used for?
Penpulimab is an FDA-approved immunotherapy used for the treatment of non-keratinizing nasopharyngeal carcinoma (NPC), both as a first-line treatment in combination with chemotherapy and as a single agent for patients who have progressed after prior therapies.
How does Penpulimab work in treating cancer?
Penpulimab targets PD-1, a protein on immune cells that helps cancer cells evade immune detection. By blocking PD-1, Penpulimab enhances the immune system’s ability to identify and attack cancer cells.
What are the common side effects of Penpulimab?
Common side effects include fatigue, nausea, rash, hypothyroidism, and diarrhea. Most side effects are manageable and temporary, but some may require medical attention.
What is the recommended dosage for Penpulimab?
For first-line NPC treatment, the recommended dose is 200 mg every 3 weeks, combined with chemotherapy. As a monotherapy for metastatic NPC, it is administered at 200 mg every 2 weeks.
How long does treatment with Penpulimab last?
Treatment duration typically lasts up to 24 months, or until disease progression or intolerable side effects occur, depending on the individual response.
Are there any serious side effects of Penpulimab?
Serious side effects may include immune-mediated reactions like pneumonitis, colitis, hepatitis, and myocarditis.
Is Penpulimab FDA-approved for other cancers?
Currently, it is FDA-approved for the treatment of non-keratinizing nasopharyngeal carcinoma (NPC), but ongoing trials are exploring its use for other types of cancer.


