Metastatic castration-resistant prostate cancer (mCRPC) remains a lethal disease despite major therapeutic advances over the past decade. Although androgen receptor pathway inhibitors (ARPIs), taxane chemotherapy, PARP inhibitors in selected populations, and PSMA-targeted radioligand therapies have extended survival, most patients eventually progress, and durable disease control after multiple lines of therapy remains uncommon. Immunotherapy with immune checkpoint inhibitors has shown limited benefit in unselected mCRPC populations, underscoring the need for alternative immune-based strategies that can overcome prostate cancer’s immunologically “cold” tumor microenvironment (Stein et al., 2025; Antonarakis et al., 2020).
Pasritamig (JNJ-78278343) represents a novel attempt to address this unmet need through T-cell redirection, a strategy that has transformed outcomes in hematologic malignancies but has historically faced substantial safety and efficacy barriers in solid tumors. By targeting human kallikrein-2 (KLK2), a prostate-lineage–restricted antigen, Pasritamig is designed to activate cytotoxic T cells selectively at the tumor site, potentially widening the therapeutic window compared with earlier T-cell engager approaches (National Cancer Institute, 2024).
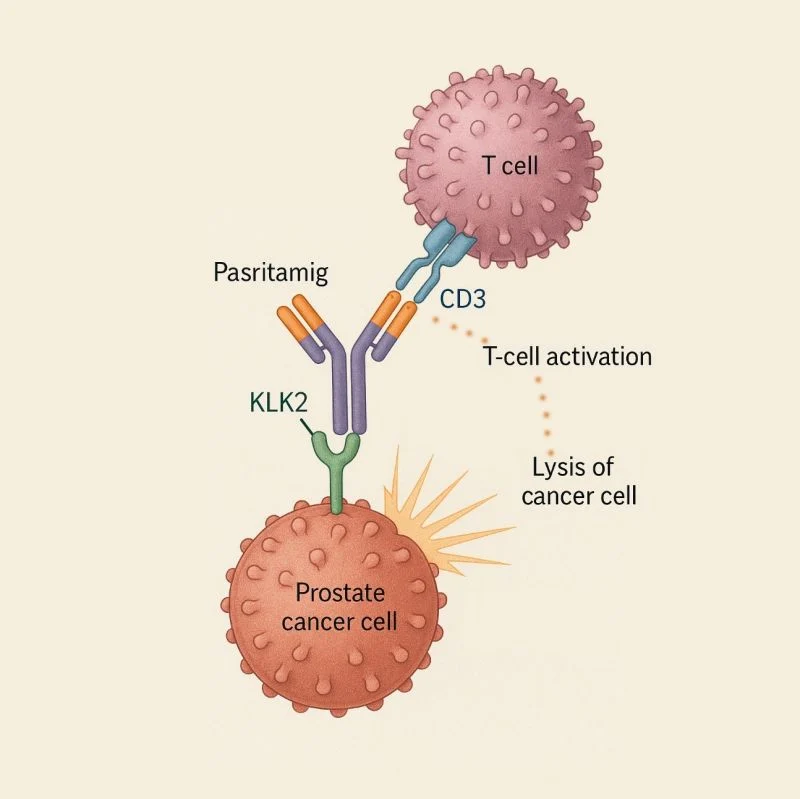
Photo: Depositphotos
Molecular Design and Mechanism of Action
Pasritamig is a humanized IgG1 bispecific antibody that simultaneously binds CD3ε on T lymphocytes and KLK2 expressed on prostate cancer cells. This dual engagement brings T cells into close proximity with tumor cells, triggering immune synapse formation, T-cell activation, and perforin/granzyme-mediated tumor cell lysis independent of major histocompatibility complex (MHC) presentation (NCI Drug Dictionary, 2024).
KLK2 is a serine protease closely regulated by androgen receptor signaling and is predominantly expressed in prostate tissue. Its restricted expression profile provides the biological rationale for using KLK2 as a target to limit off-tumor toxicity, a key challenge that has constrained the development of T-cell engagers in solid tumors (Stein et al., 2025). Preclinical studies demonstrated that KLK2-directed T-cell redirection induces potent cytotoxicity in KLK2-expressing prostate cancer models while sparing non-prostatic tissues, supporting clinical translation (Baldini et al., 2025).
Early Clinical Development and Phase I Study Design
The first-in-human clinical evaluation of Pasritamig was conducted in a multicenter Phase I trial (NCT04898634) enrolling patients with heavily pretreated mCRPC. The primary objectives were to assess safety, dose-limiting toxicities, and pharmacokinetics, while secondary and exploratory objectives included antitumor activity and immune pharmacodynamics (Stein et al., 2025).
Patients enrolled in the trial had received a median of approximately four prior systemic therapies, including universal exposure to androgen receptor pathway inhibitors and high rates of prior taxane chemotherapy. Both intravenous and subcutaneous formulations were explored early in development, with multiple step-up dosing schedules tested to mitigate cytokine release syndrome (CRS), a known class effect of T-cell–redirecting therapies (Baldini et al., 2025).
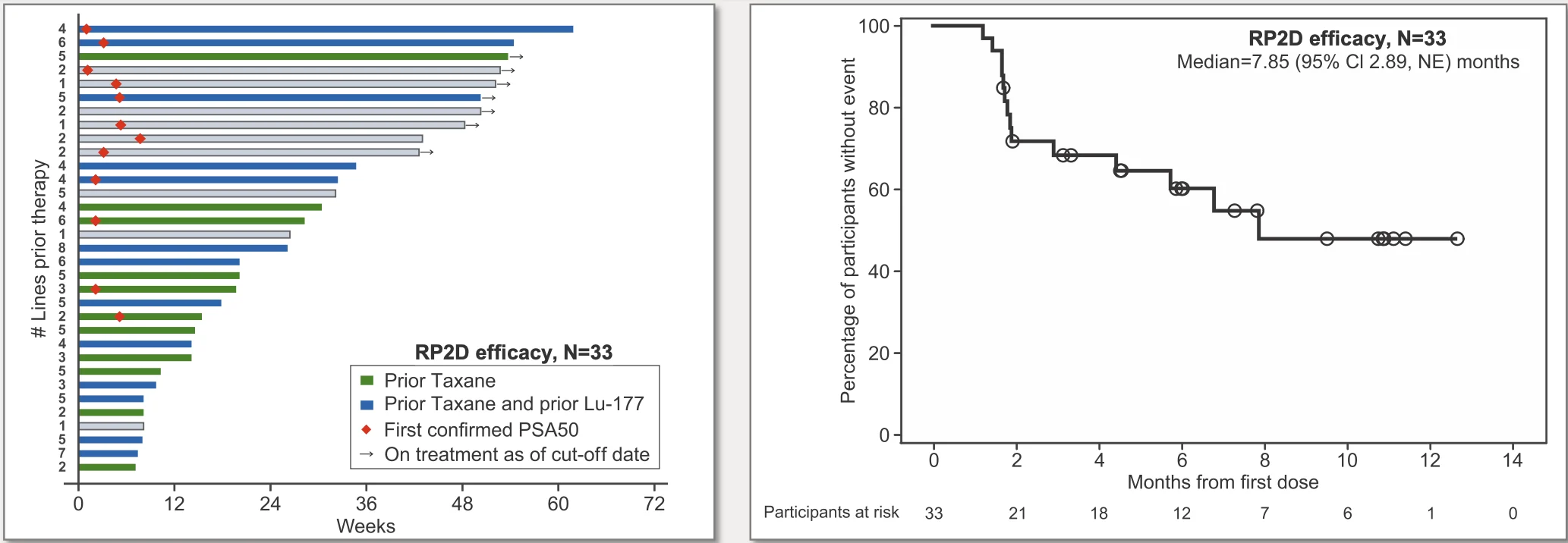
Photo: Depositphotos
Dose Optimization and Outpatient-Friendly Administration
A key achievement of the Phase I program was the identification of a recommended Phase II dose (RP2D) that balances immune activation with tolerability. The selected regimen incorporated step-up dosing (3.5 mg on Day 1 and 18 mg on Day 8), followed by a target dose of 300 mg intravenously on Day 15 and subsequent every-6-week (Q6W) maintenance dosing (Johnson & Johnson, 2025).
This extended dosing interval is particularly notable in the context of T-cell engagers, which often require weekly or biweekly administration. A Q6W schedule has important implications for patient convenience, healthcare resource utilization, and feasibility in outpatient oncology settings, especially for older mCRPC populations with multiple comorbidities (Stein et al., 2025).
Safety Profile and Cytokine Release Syndrome
Safety has historically been the major limiting factor for T-cell–redirecting therapies in solid tumors. In the Pasritamig Phase I trial, treatment-related adverse events were common but largely manageable. Importantly, at the RP2D, cytokine release syndrome occurred in fewer than 10% of patients and was exclusively Grade 1, with no reported Grade ≥3 CRS events (Baldini et al., 2025).
Other immune-related toxicities, including neurotoxicity, were infrequent and generally low grade. These findings suggest that careful dose engineering and step-up administration can substantially reduce acute immune toxicity without abrogating antitumor activity, a critical requirement for broader clinical adoption (Stein et al., 2025).
Early Signals of Antitumor Activity
Although the Phase I study was not designed to assess efficacy definitively, encouraging signals of clinical activity were observed. Among patients treated at the RP2D on the Q6W schedule, approximately 42% achieved a ≥50% decline in prostate-specific antigen (PSA) levels, a commonly used biomarker of response in mCRPC (Johnson & Johnson, 2025).
Radiographic outcomes further supported biological activity, with a reported median radiographic progression-free survival of 7.9 months in this heavily pretreated population. At the time of data cutoff, roughly one-fifth of patients remained on therapy, suggesting the potential for durable disease control in selected individuals (Baldini et al., 2025).
While cross-trial comparisons should be interpreted cautiously, these outcomes are notable given the advanced disease state and extensive prior treatment exposure of the enrolled population (Stein et al., 2025).
Translational Insights and Immune Pharmacodynamics
Translational analyses presented alongside the clinical data explored T-cell activation markers, cytokine profiles, and pharmacokinetic-pharmacodynamic relationships. These studies demonstrated dose-dependent T-cell engagement and activation without sustained systemic cytokine elevations, supporting the biological plausibility of intermittent, high-dose administration rather than continuous exposure (van Aken et al., 2025).
Such findings are particularly relevant in solid tumors, where excessive or prolonged immune activation can lead to toxicity without improving efficacy. The Pasritamig program highlights the importance of integrating translational immunology into early-phase clinical development to optimize therapeutic index (van Aken et al., 2025).
Regulatory Progress and Ongoing Phase III Development
Based on the totality of early clinical and translational data, Pasritamig has received FDA Fast Track designation for the treatment of mCRPC, reflecting the significant unmet medical need in this population and the drug’s potential to address it (Urology Times, 2025).
Multiple Phase III trials are currently underway to further define the role of Pasritamig in mCRPC, including randomized studies evaluating Pasritamig-based regimens versus placebo or standard of care, as well as combination strategies with established agents such as docetaxel (ClinicalTrials.gov, 2025). These studies will be critical in determining whether early PSA and rPFS signals translate into meaningful overall survival benefits.
Potential Clinical Positioning and Future Directions
If ongoing Phase III trials confirm a favorable balance of efficacy and safety, Pasritamig could emerge as a novel immune-based option for patients with mCRPC who have exhausted standard therapies. Its prostate-restricted target, manageable CRS profile, and infrequent dosing schedule differentiate it from earlier T-cell engager approaches and may enable use beyond highly specialized centers (Stein et al., 2025).
Future research will need to clarify optimal patient selection, including the role of KLK2 expression levels, tumor heterogeneity, and immune contexture in predicting response. Combination strategies, particularly with chemotherapy or other immune-modulating agents, may further enhance activity while maintaining tolerability (Antonarakis et al., 2020).
Conclusion
Pasritamig (JNJ-78278343) represents a promising step forward in the application of T-cell–redirecting therapies to solid tumors, specifically metastatic castration-resistant prostate cancer. Early-phase data demonstrate that KLK2-directed T-cell engagement can produce clinically meaningful antitumor activity with a manageable safety profile when supported by rational dose and schedule optimization. As Phase III trials mature, Pasritamig will help determine whether T-cell engagers can finally secure a durable role in the treatment landscape of advanced prostate cancer.


