Ivonescimab: Evaluating Safety, Tolerance, and Efficacy of a Novel Bispecific Antibody Treatment
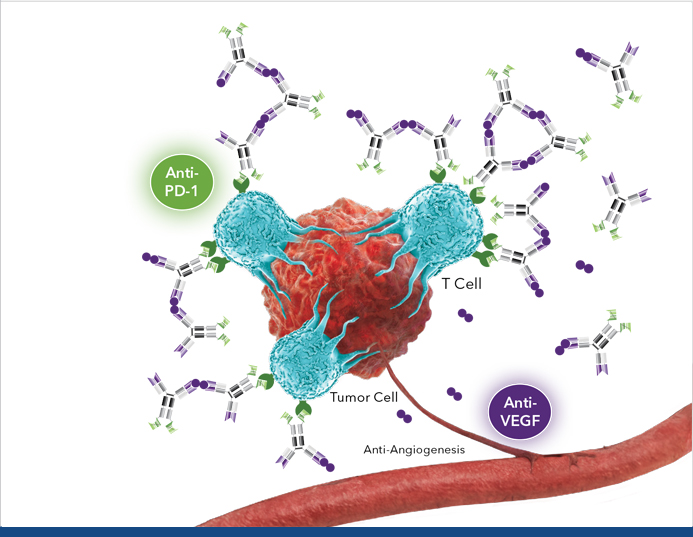
Ivonescimab, a humanized IgG1 bispecific anti-PD-1/VEGF antibody, has been evaluated for safety and efficacy in patients with extensive-stage small cell lung cancer (ES-SCLC) when combined with etoposide and carboplatin. This study involved administering ivonescimab at doses of 3 mg/kg, 10 mg/kg, or 20 mg/kg every three weeks for up to four cycles, followed by maintenance therapy.
This study aims to assess the safety and tolerance of ivonescimab when combined with etoposide and carboplatin as a first-line treatment. Given the high unmet need for effective therapies in ES-SCLC, this investigation not only seeks to evaluate the objective response rate (ORR) but also to establish a new standard of care that could significantly improve outcomes for patients facing this aggressive cancer. Through a comprehensive analysis of treatment responses and adverse events, the study aims to contribute valuable insights into the potential of ivonescimab in enhancing cancer care.
Study Overview:
- Participants: 35 patients enrolled between April 23, 2021, and December 2, 2021.
- Follow-Up: Median follow-up of 13.3 months (range: 0.3-28.5 months) as of October 25, 2023.
- Primary Endpoints: Safety and objective response rate (ORR).
Results:
- Overall Response Rate (ORR): 80% with a disease control rate of 91.4%.
- Dose-specific ORR:
- 3 mg/kg: 66.7%
- 10 mg/kg: 90.9%
- 20 mg/kg: 76.2%
- Adverse Events: Grade ≥3 treatment-related adverse events (TRAEs) occurred in 60% of patients, including:
- Decreased neutrophil count (22.9%)
- Decreased white blood cell count (14.3%)
- Anemia (14.3%)
- TRAEs leading to death were reported in two patients (5.7%).
Conclusions:
The combination of ivonescimab with etoposide and carboplatin demonstrated promising antitumor activity and was well-tolerated in patients with ES-SCLC, indicating its potential as a viable first-line treatment option.
Authors: Zhiwei Chen, Lin Wu, Qiming Wang, Yan Yu, Xianling Liu, Rui Ma, Tao Li, Yan Li, Xia Song, Lin Li, Wei Zhao, Qiaoyun Wang, Xiao Xu, Shun Lu,

“Phase Ib study of ivonescimab (PD-1 VEGF bispecific) with first-line carboplatin + etoposide in SCLC. In 35 pts, RR 80%, PFS 6.9m, OS 14.5m, G3+ TRAEs in 60% with most frequent being neutropenia (23%).”
“Ph Ib trial of Ivonescimab+Carbo/Etop in 1L ES SCLC | Journal of Thoracic Oncology:
– 35 patient]ts (3, 10, 20mg/kg dose levels)
– ORR 80%, DCR 91.4%
– G3+ TRAEs 60% (mainly low ANC, low WBC, low Hb)
Another application of potential interest for the PD-1/VEGF bispecific.”
“Ivonescimab with etoposide and carboplatin as 1st-line treatment for extensive-stage SCLC.
Phase Ib (N=35)
ORR and DCR: 80% and 91.4%, respectively
PFS 6.9m, OS 14.5m (1y OS 72%)
Grade ≥3 TRAEs: 60% (neutropenia 23%).”
More posts featuring Immunotherapy on oncodaily.com