Daratumumab, an anti-CD38 monoclonal antibody, has been approved for treating multiple myeloma.
On December 9th results of AQUILA Trial were published at the NEJM. Resents of Trial were presented at ASH 2024 meeting.
Daratumumab or Active Monitoring for High-Risk Smoldering Multiple Myeloma
Authors: Meletios A. Dimopoulos, Peter M.Voorhees, Fredrik Schjesvold, Yael C. Cohen, Vania Hungria, Irwindeep Sandhu, Jindriska Lindsay, Ross I. Baker, Kenshi Suzuki, Hiroshi Kosugi, Mark-David Levin, Meral Beksac, Keith Stockerl-Goldstein, Albert Oriol, Gabor Mikala, Gonzalo Garate, Koen Theunissen, Ivan Spicka, Anne K.Mylin, Sara Bringhen, Katarina Uttervall, Bartosz Pula, Eva Medvedova, Andrew J. Cowan, Philippe Moreau, Maria-VictoriaMateos, Hartmut Goldschmidt, Tahamtan Ahmadi, Linlin Sha, Annelore Cortoos, Eva G. Katz, Els Rousseau, Liang Li, Robyn M. Dennis, Robin Carson, and S. Vincent Rajkumar for the AQUILA Investigators.

Methods
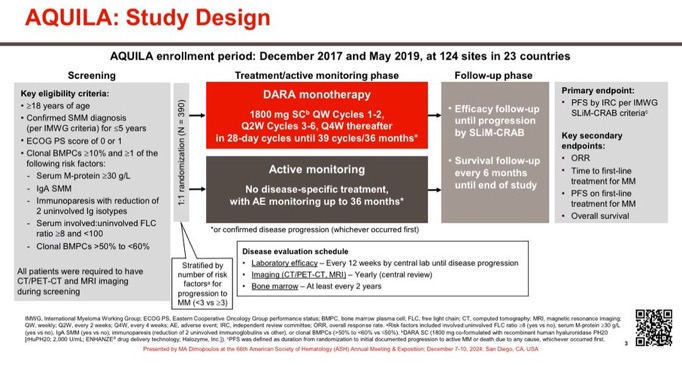
In this phase 3 trial, patients with high-risk smoldering multiple myeloma were randomly assigned to receive either subcutaneous daratumumab monotherapy or active monitoring.
Treatment continued for up to 39 cycles, 36 months, or until disease progression, whichever occurred first.
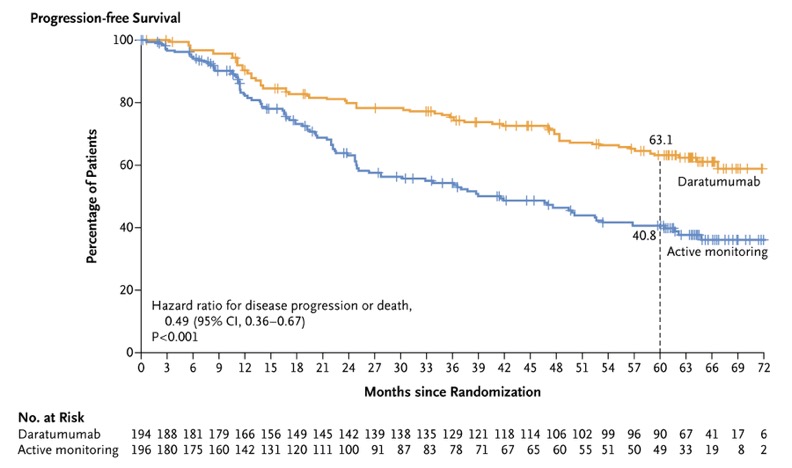
The primary endpoint was progression-free survival, with disease progression assessed by an independent review committee based on International Myeloma Working Group criteria.
Results
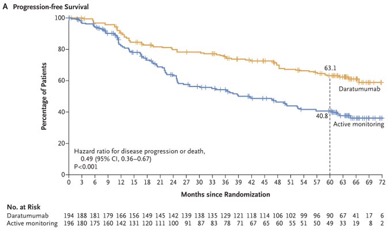
A total of 390 patients were enrolled, with 194 in the daratumumab group and 196 in the active-monitoring group. After a median follow-up of 65.2 months, the risk of disease progression or death was 51% lower in the daratumumab group compared to the active-monitoring group (hazard ratio, 0.49; 95% CI, 0.36 to 0.67; P<0.001).
At 5 years, progression-free survival was 63.1% for the daratumumab group and 40.8% for the active-monitoring group.
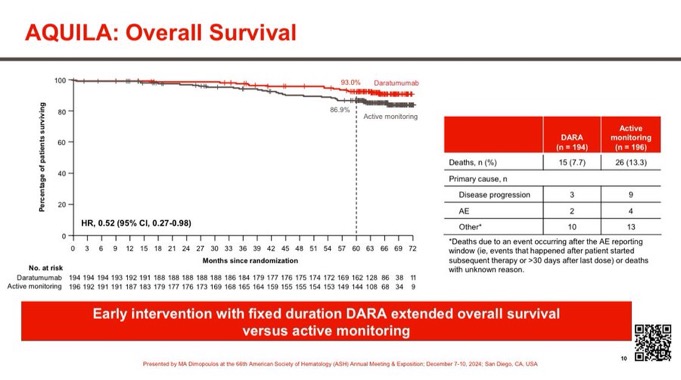
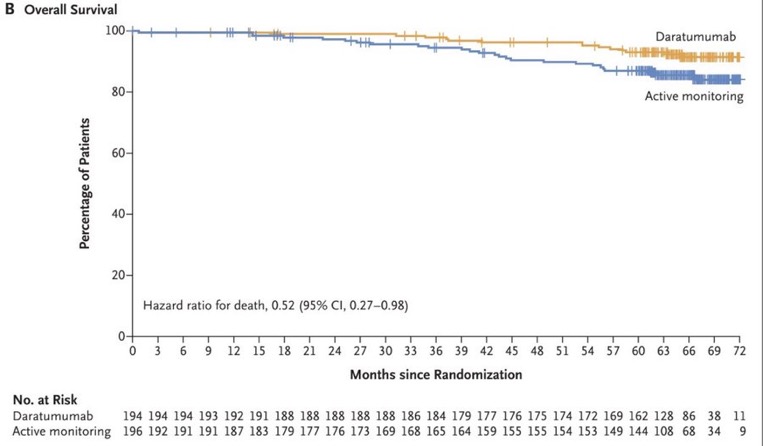
Fifteen patients (7.7%) in the daratumumab group and 26 patients (13.3%) in the active-monitoring group died (hazard ratio, 0.52; 95% CI, 0.27 to 0.98). The 5-year overall survival rate was 93.0% for the daratumumab group and 86.9% for the active-monitoring group.
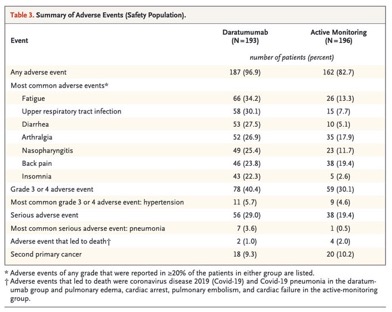
The most common grade 3 or 4 adverse event was hypertension, occurring in 5.7% of the daratumumab group and 4.6% of the active-monitoring group. Treatment discontinuation due to adverse events occurred in 5.7% of the daratumumab group, with no new safety concerns identified.
NEJM:
“Presented at ASH24:
Among patients with smoldering multiple myeloma at a high risk for progression, progression-free survival at 5 years was 63.1% with daratumumab monotherapy, as compared with 40.8% with active monitoring. Full AQUILA trial results.”
“Just out: Paradigm changing AQUILA randomized trial in high risk smoldering myeloma.
Daratumumab significantly prolongs time to active myeloma and overall survival. Proud to be a lead investigator of this trial.
Main Findings Daratumumab prolongs:
-Time to active myeloma
-Time to CRAB
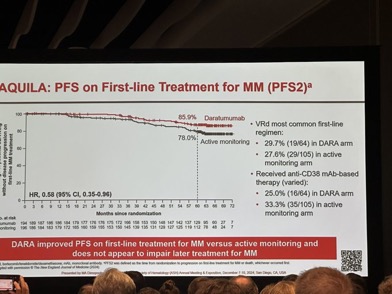
-PFS2
-Overall Survival
Plus: Low toxicity, no detriment to QOL, and limited duration therapy.
390 patients with high risk SMM randomized. Largest trial in SMM ever conducted.
Limited duration therapy.
Significant improvement in time to progression to active myeloma or death with Dara. Benefit seen in almost all subgroups.
64% single agent response rate with Dara. Significant prolongation of time to needing active myeloma therapy.
Improved PFS2 No adverse impact of Dara therapy on subsequent outcomes.
Superior Overall Survival with Dara. HR 0.52 95% CI 0.27-0.98.
Good safety profile. Similar numbers despite longer follow up.
There is a 10% absolute higher risk of grade 3-4 AEs some of which is related to longer period on study in Dara arm.
No detriment on quality of life measurements.
Conclusions.
In high risk smoldering myeloma,
Daratumumab prolongs:
-Time to active myeloma
-Time to CRAB
-PFS2
-Overall Survival
Low toxicity, no detriment to QOL, and limited duration therapy.”
“Pepr of the Day: AQUILA study shows patients w/ high-risk smoldering myeloma who get subcu daratumumab monotherapy had a significantly lower risk of progression to active myeloma or death and trend to higher overall survival (median NR).”
“AQUILA trial data presented by Prof Meletios Dimopoulos at ASH24.
390 pts with high-risk SMM randomised to 36 m of daratumumab (n=194) or active monitoring (n=196)
DARA reduced risk of MM progression or death by 51% and improved 5-year OS”
“This is likely the breaking news of the day at ASH24
Aquila soars high,
Hope in smoldering shadows,
New light breaks the dark.”
The International Academy for Clinical Hematology (IACH):
“Breaking News: the AQUILA study from ASH24.
Talk presented by Vincent Rajkumar.
Click to hear the Breaking News.
IACH community extends its congratulations to all stakeholders for the remarkable achievement with the AQUILA trial data in smoldering myeloma. This groundbreaking work will undoubtedly have a profound impact on patient care and clinical strategies moving forward.”

“AQUILA Trial is out! Important to note that ~30% of patients received a clearly suboptimal 1st line anti-myeloma treatment (no PI+IMiD- based triplet or CD38-based combos), which makes the OS signal unreliable in the current climate of Quad (Dara/Isa-VRd) frontline therapy!
Also, just ~30% had received anti-CD38 mAb-based frontline therapy!”
“QUILA: Daratumumab x 3 years vs. active surveillance for smoldering myeloma
– All patients had advanced imaging (CT/PET/MRI during screening and yearly)
– Primary endpoint: PFS per IMWG SLiM-CRAB criteria
– Secondary endpoints: included OS (see image for stats)
– Only about 40% of patients were considered high-risk by 2/20/20 criteria.
– PFS: significant benefit for daratumumab
– OS: HR 0.52 (95% CI 0.27-0.98) though the study not powered for this.
What about progression to morbid events?
– Doesn’t appear that active monitoring led to significant morbid events among patients who progressed.
– Adverse events: The incidence of grade 3 or 4 infections was 16.1% in the dara group and 4.6% in the active-monitoring group
– As for post-protocol therapies, VRd was most common in both groups. Not much dara used frontline in the active surveillance group. Did that have an impact?
I am still not convinced about daratumumab here
– Did not answer the question of whether waiting to start Dara-VRd frontline is better (or any dara-based regimen).”
“Some key slides on the AQUILA trial of Dara in SMM just presented ASH24.
So hard to interpret
Reassuringly most progression events were SLiM or anaemia
If only high quality first line treatment (ie VRd or DVRd) had been included/mandated…”
“AQUILA randomized trial in high risk smoldering myeloma ASH24.
Daratumumab significantly prolongs time to active myeloma and overall survival.
This could represent a significant shift in treatment for plasma cell disorders! Well done, Vincent Rajkumar.”
“Results of the AQUILA trial showed that the use of subcutaneous daratumumab, an anti-CD 38 monoclonal antibody, as monotherapy for patients with high-risk smoldering multiple myeloma (HRSMM), was associated with a significantly lower risk of progression to active MM or death and higher overall survival than active monitoring.
Hypertension was observed in the study as an adverse effect in 5.7% of patients receiving daratumumab leading to treatment discontinuation. Although an effective monotherapy is a huge stride in managing HRSMM vs. active monitoring, it is fundamental to weigh the advantages and disadvantages of this treatment, given the serious adverse effects of daratumumab and its accessibility to patients.”
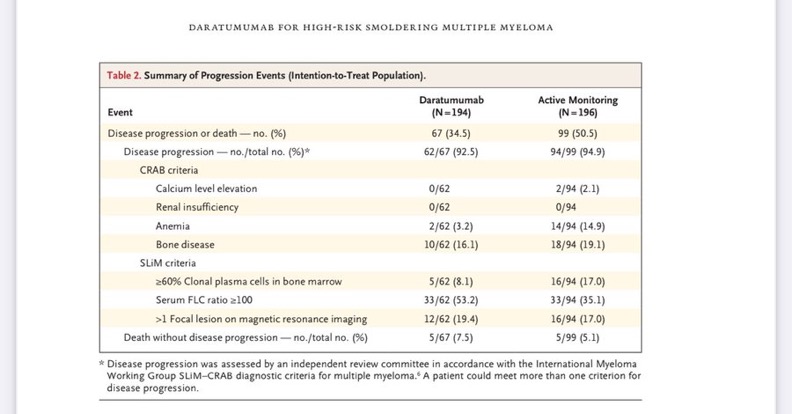
“AQUILA trial
Progression events (very important initial observations
No renal insufficiency in observation arm
Even with treatment , pts on Dara have progression per SLIM criteria
Bone disease progression is similar between both arms
2.5 % more death with no progression in Dara arm.
About half of pts who were in control arm didn’t receive therapy
All over the utilization of Dara in control arm is low
Nice to provide this data in supplemental.”
“An incredible ASH for patients with HR-SMM! AQUILA trial shows that daratumumab improves PFS and OS and kudos to Vincent Rajkumar, Thanos Dimopoulos for leading this important study. Next frontier is cure and we will keep working together to find it!
Irene Ghobrial, Betsy O’Donnell, Marivi Mateos, Sagar Lonial ++ many others working on precursor disease!”


