Amol Akhade, Consultant Medical Oncologist at the Suyog Cancer Clinics, shared a post on LinkedIn:
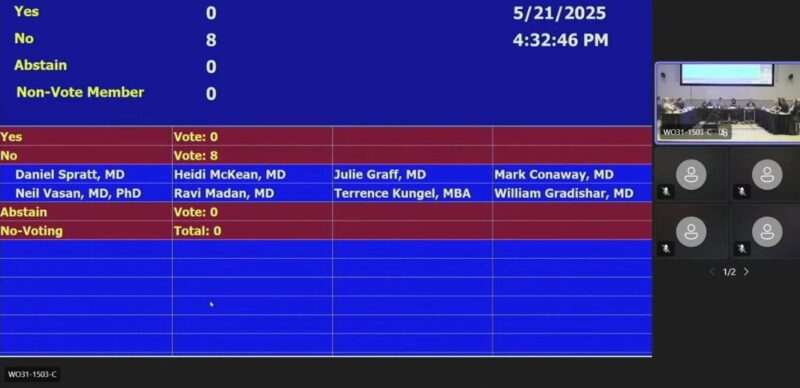
“ODAC Votes 8 – 0 Against Talazoparib + Enzalutamide in non-HRRm mCRPC
In a unanimous 8–0 decision, the FDA’s Oncologic Drugs Advisory Committee (ODAC) voted against expanding the label for talazoparib + enzalutamide to include patients with metastatic castration-resistant prostate cancer (mCRPC) who lack HRR mutations.
Key reasons behind the rejection:
– No prespecified statistical analysis for the non-HRRm subgroup
– Immature overall survival (OS) data
– Absence of prospective biomarker stratification
– Lack of supporting data from prior PARP inhibitor trials in non-HRRm patientsConcern that observed benefits may be due to chance.
The committee expressed concern that approving this broad label would set a precedent that rewards inadequate methodology and undermines scientific rigor.
While trends in rPFS were noted (not reached vs. 21.9 months), the absence of rigorous subgroup validation made the case unconvincing.
Takeaway:
The combination remains approved only for HRR-mutated mCRPC Biomarker-driven precision oncology remains the benchmark for regulatory approval Caution urged against exploratory claims without statistical and biological justification
This decision reinforces that regulatory approval must be driven by data, not extrapolation.”
More posts featuring Amol Akhade on OncoDaily.