Vinay Prasad, Professor of Epidemiology and Biostatistics at the University of California, San Francisco, shared a post on X:
“Just out in JAMA Oncology: We look at all Adjuvant and Neoadjuvant drugs approved by FDA in last 5 years.
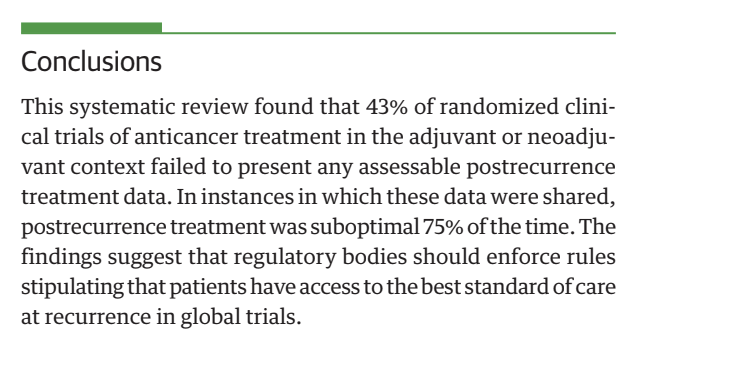
43% of the time trials DON’T SAY what control arm patients get at relapse 75% of the time it is SUBOPTIMAL. This means OS is unreliable.
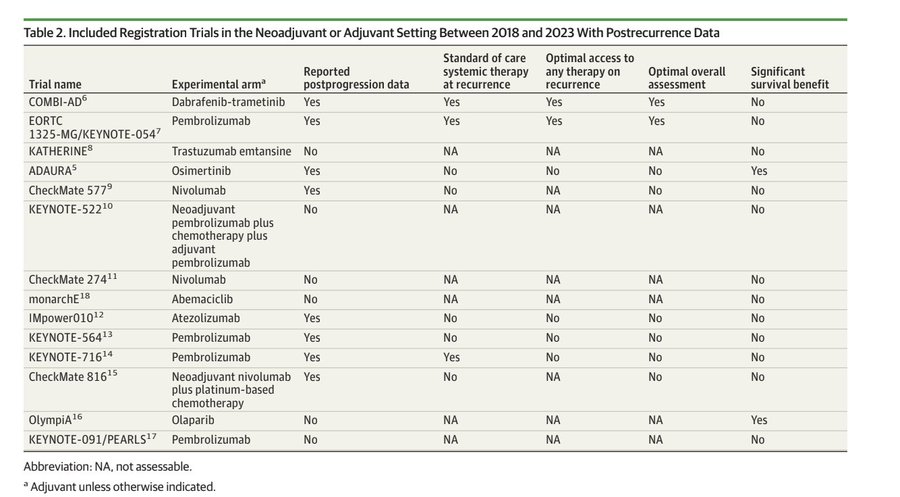
These are the drugs and trials we looked at. Very new, costly, toxic and glamorous agents, praised by KOLs All improve DFS/EFS/RFS but only 2 improved OS at the time, now it is 4. But here is the kicker:
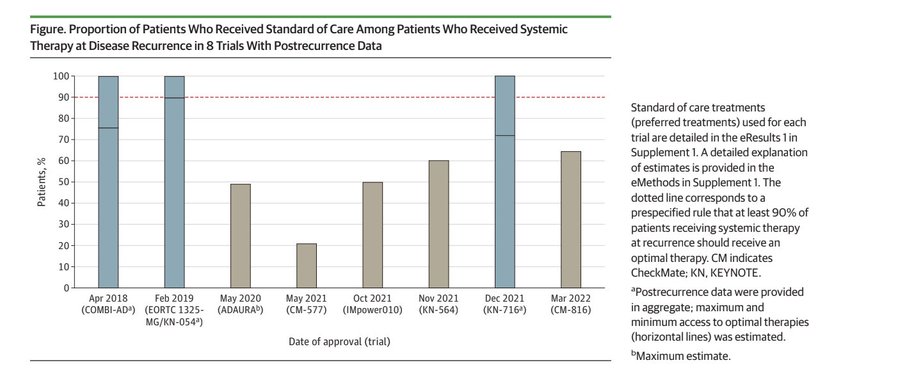
The rate of standard of care upon progression is very low. The blue bars indicate reporting so poor, we have only an upper and lower bound of what percent get standard of care.
This means, practically, ADAURA – We don’t know its better to give adjuvant to stage Ib than reserve for relapse. CHECKMATE-564 – We don’t its better to reserve pembro for relapse the list goes on.
This means we spend BILLIONS on drugs, and we don’t actually have trials that ask the relevant question: Is it better to give early, and to some people who may already be cured, or to just keep doing what we are doing. An evidence based disaster.
FDA allows this to happen, and has the ability to demand better studies. Sad to see investigators participate.
Here is a link to updated figures. Timothée Olivier did all the hard work.”
Source: Vinay Prasad/X