
Adagrasib (Krazati) Patient Guide 2025: Targeted Therapy for Lung and Colorectal Cancer
Adagrasib is a new oral cancer treatment that offers hope to people with specific genetic mutations in their cancer. It has been approved by the U.S. Food and Drug Administration (FDA) for use in non-small cell lung cancer (NSCLC) and colorectal cancer (CRC) that carry the KRAS G12C mutation. This medication is used after patients have already received other cancer treatments. Adagrasib is part of a new generation of targeted therapies that aim to block the growth of cancer by acting directly on the genetic drivers of the disease.
What Is Adagrasib and How Does It Work?
Adagrasib, known by its brand name Krazati, is a type of medicine known as a KRAS G12C inhibitor. KRAS is a protein that plays a key role in controlling how cells grow and divide. When it becomes mutated—especially in the form known as KRAS G12C—it can cause cells to grow uncontrollably, leading to cancer. Adagrasib works by locking this abnormal KRAS G12C protein into an inactive state. This blocks the signals that tell cancer cells to grow and survive.
Unlike earlier cancer drugs that couldn’t target KRAS, adagrasib is designed to fit into the mutated protein like a key in a lock. It keeps KRAS turned off for longer periods of time, which helps stop the cancer from spreading. It is especially useful for cancers that have become resistant to other treatments.
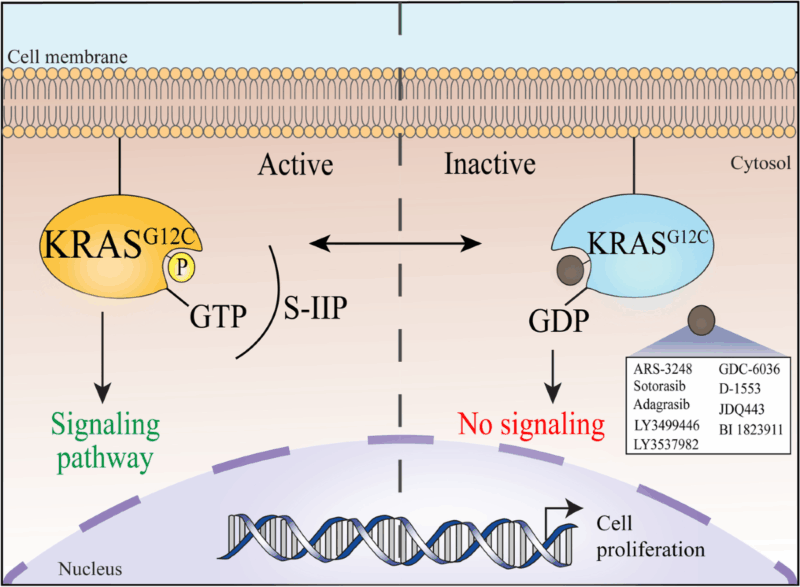
Source: Journal of Experimental & Clinical Cancer Research
What Cancers Does Adagrasib Treat?
Adagrasib is approved for adults with advanced or metastatic non-small cell lung cancer who have the KRAS G12C mutation and have already been treated with at least one other systemic therapy. It is also approved for people with advanced colorectal cancer that has the same KRAS G12C mutation. In colorectal cancer, adagrasib is used together with another drug called cetuximab after the patient has already received chemotherapy. Before prescribing this treatment, doctors will test the cancer to make sure the KRAS G12C mutation is present.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Adagrasib was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
How Effective Is Adagrasib? What Clinical Trials Show
Clinical trials have shown that adagrasib is effective in shrinking tumors and slowing disease progression in patients with KRAS G12C–mutated cancers.
In patients with non-small cell lung cancer, results from the KRYSTAL-1 trial showed that adagrasib helped shrink tumors in about 43% of patients. Most of these patients had already been treated with both chemotherapy and immunotherapy. The response lasted for a median of 8.5 months, and patients lived without cancer worsening for a median of 6.5 months. The overall survival was 12.6 months. Even patients with brain metastases saw benefits, with some showing significant tumor shrinkage in the brain.
You can read about Non-Small Cell Lung Cancer: Causes, Symptoms, Diagnosis, Treatment Options, and Latest 2025 Advances in Targeted and Immunotherapy on OncoDaily.
In colorectal cancer, the KRYSTAL-1 trial tested adagrasib in combination with cetuximab. This study showed a 34% tumor response rate, meaning about one in three patients had a measurable benefit. The response lasted around 5.8 months. Based on this, the FDA granted the combination approval with a priority review and breakthrough therapy status.
Adagrasib Side Effects and Management
Krazati, like many cancer treatments, can cause side effects. While not everyone experiences them, many patients report symptoms that range from mild to more serious. Knowing what to expect can help patients and caregivers recognize early signs and manage symptoms effectively.
Common side effects of adagrasib
The most frequently reported side effects include nausea, diarrhea, vomiting, fatigue, and decreased appetite. These symptoms often begin early in the course of treatment and may improve over time. Some patients also report constipation, cough, or mild elevations in liver enzymes. While these side effects can be uncomfortable, they are usually manageable with medications and lifestyle adjustments.
Less common but serious side effects
A smaller number of patients may develop more serious side effects. These include lung inflammation (pneumonitis), liver toxicity, and heart rhythm changes such as QT interval prolongation. These complications are less common but may require prompt medical attention, temporary interruption of treatment, dose reduction, or stopping the drug entirely. Routine lab tests and clinical checkups help detect these issues early.
How to manage side effects of adagrasib?
Your healthcare team will help you manage side effects throughout your treatment. Nausea and vomiting can often be controlled with anti-nausea medications, while diarrhea may respond to antidiarrheal drugs and increased hydration. Fatigue can be addressed by adjusting daily routines and ensuring good nutrition. Serious side effects like pneumonitis or liver enzyme elevations will be monitored closely and may prompt treatment changes. Always communicate openly with your medical team about any new or worsening symptoms.

How is Krazati administered?
Adagrasib is taken by mouth as an oral tablet. Patients are usually instructed to take it twice a day, around the same time each day, with or without food. The tablets should be swallowed whole with water, not chewed, crushed, or broken. Patients should follow their doctor’s instructions carefully and avoid taking a missed dose unless there is sufficient time before the next one. It’s important to stay consistent with the schedule to maintain steady levels of the medication in the body.
What is the recommended dose of Adagrasib?
The FDA-approved recommended dose of adagrasib is 600 mg taken twice daily. This dosage was established based on clinical trials, including the KRYSTAL-1 and KRYSTAL-10 studies, which showed effective tumor response and manageable side effects at this level. However, your healthcare provider may adjust the dose or recommend temporary pauses in treatment depending on how your body tolerates the drug or if you experience any side effects.
Learn more about Colorectal Cancer: Epidemiology, Pathogenesis, Diagnosis, and Therapeutic Advances on OncoDaily.
How Is Adagrasib Processed by the Body?
After you take adagrasib, it is absorbed into your bloodstream and broken down mainly in the liver by enzymes such as CYP3A4. Its half-life—meaning the time it takes for half the drug to leave the body—is about 23 hours. Most of the drug is eliminated through your stool, with a smaller amount leaving the body through urine. This long half-life helps the drug stay active between doses, offering continuous protection against cancer growth.
What Should You Avoid While Taking Adagrasib?
Certain substances and habits can interfere with how adagrasib works. You should avoid grapefruit and grapefruit juice, as they can affect how the drug is processed in the liver. Avoid taking supplements or medicines that act on liver enzymes without talking to your doctor first—especially drugs like rifampin or herbal products like St. John’s wort. Smoking can also change how your body handles the medication and should be avoided.
Because adagrasib may cause fatigue or dizziness, it’s best not to drive or operate machinery until you know how the drug affects you.
What Is the Long-Term Outlook?
Adagrasib is not a cure, but it can offer months of disease control and symptom relief, especially in cancers with limited treatment options. In lung cancer, patients receiving adagrasib have lived over a year on average following treatment. In colorectal cancer, it has shown durable responses when combined with cetuximab. Some patients experience long-lasting benefits, and ongoing research is working to make these benefits even greater.
Real-Life Effectiveness
Outside of clinical trials, adagrasib has also shown promise in real-world settings. Patients who have already been through multiple treatments are seeing tumor shrinkage, symptom improvement, and better quality of life. Its convenient oral administration and tolerable side effect profile make it easier for patients to stay on therapy and remain active in their daily lives.
Ongoing Clinical Trials and Future Research
Adagrasib is being tested in several ongoing clinical trials to better understand how it works alone or in combination with other cancer therapies. In the KRYSTAL-7 trial, researchers tested adagrasib together with the immunotherapy drug pembrolizumab in patients with advanced lung cancer who had not received any prior treatment. This combination resulted in a response rate of 49%, with a median response duration of 12.6 months and overall survival of 18.1 months.
Another study, KRYSTAL-21, is comparing different ways of taking adagrasib—either with food or without—to see which option gives better results and fewer side effects. These ongoing trials may help expand the use of adagrasib and improve outcomes for more patients in the future.
If you’re a healthcare provider, access the professional version here.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
