Spencer Knight, Senior Business Consultant of Cell and Gene Therapy at Charlton Morris, shared on LinkedIn:
“5 approvals in the last 6 months!
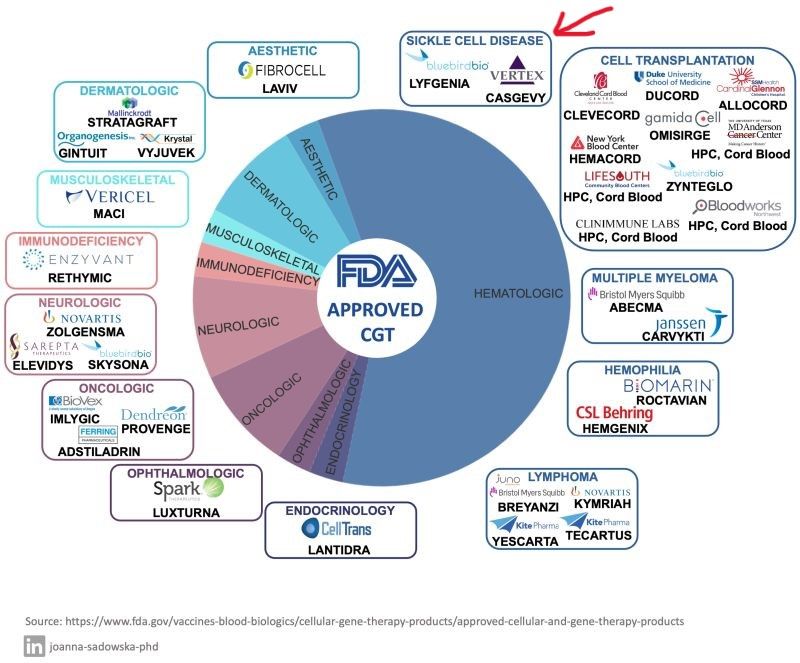
Updated graphic below reflects the two new FDA approvals achieved on Friday by Vertex Pharmaceuticals/CRISPR Therapeutics and bluebird bio in sickle cell disease. From 32 to now 34 approvals since our last post.
Here’s the current 34 approval breakdown by therapeutic area:
𝐇𝐚𝐞𝐦𝐚𝐭𝐨𝐥𝐨𝐠𝐲 (20 products):
– 2 for the treatment of multiple myeloma
– 4 for the treatment of lymphoma
– 2 for haemophilia
– 10 for cell transplant-based therapies
– 2 recently approvals for sickle cell disease: 𝐂𝐀𝐒𝐆𝐄𝐕𝐘 and 𝐋𝐘𝐅𝐆𝐄𝐍𝐈𝐀.
𝐄𝐧𝐝𝐨𝐜𝐫𝐢𝐧𝐨𝐥𝐨𝐠𝐲 (1 product).
𝐎𝐩𝐡𝐭𝐡𝐚𝐥𝐦𝐨𝐥𝐨𝐠𝐲 (1 product).
𝐎𝐧𝐜𝐨𝐥𝐨𝐠𝐲 (3 products).
𝐍𝐞𝐮𝐫𝐨𝐥𝐨𝐠𝐲 (3 products).
𝐈𝐦𝐦𝐮𝐧𝐨𝐝𝐞𝐟𝐢𝐜𝐢𝐞𝐧𝐜𝐲 (1 product).
𝐌𝐮𝐬𝐜𝐮𝐥𝐨𝐬𝐤𝐞𝐥𝐞𝐭𝐚𝐥 (1 product).
𝐃𝐞𝐫𝐦𝐚𝐭𝐨𝐥𝐨𝐠𝐲 (3 products).
𝐀𝐞𝐬𝐭𝐡𝐞𝐭𝐢𝐜 𝐚𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧𝐬 (1 product).
This diverse range of approved products underscores the expanding impact of CGT across various medical fields.
34 approvals is certainly positive, however, our industry MUST address manufacturing challenges early in the development process to 𝐞𝐧𝐬𝐮𝐫𝐞 𝐭𝐡𝐞 𝐜𝐨𝐦𝐦𝐞𝐫𝐜𝐢𝐚𝐥 𝐯𝐢𝐚𝐛𝐢𝐥𝐢𝐭𝐲 of these therapies. Thanks to Jason Foster and Sanjay Srivastava for recently speaking on this.
𝘞𝘩𝘢𝘵 𝘯𝘦𝘦𝘥𝘴 𝘵𝘰 𝘣𝘦 𝘢𝘥𝘥𝘦𝘥? Thanks again for allowing me to share this and hope you liked the red arrow contribution lol – Joanna Sadowska.
P.S. – Want to stay ahead of the biotech revolution? Sign up for FREE to this week’s 𝐂𝐆𝐓𝐰𝐞𝐞𝐤𝐥𝐲 newsletter and discover the latest breakthroughs and advancements, here.”
Source: Spencer Knight/LinkedIn