At the ESMO Breast Cancer Congress 2025 Dr. Budhi S. Yadav (Chandigarh, India) presented finding from phase III HYPART trial comparing 1-week adjuvant RT vs. 2-week adjuvant RT in high risk patients with breast cancer.
What Is the HYPART Trial?
The HYPART study is a Phase III randomized trial investigating shorter courses of adjuvant radiotherapy for patients with high-risk breast cancer. Specifically, it aimed to compare a hypofractionated (shorter) 1-week radiotherapy schedule against a standard 2-week schedule in patients who have undergone either mastectomy or breast-conserving surgery. It aimed to compare a hypofractionated (shorter) 1-week radiotherapy schedule against a standard 2-week schedule in patients who have undergone either mastectomy or breast-conserving surgery.
Trial Endpoints and Translational Aims
This specific report from the HYPART study focused on evaluating and comparing the acute toxicity profiles of the 1-week and 2-week radiotherapy schedules. The analysis presented here aimed to determine if the shorter treatment duration impacted the immediate side effects experienced by patients. Details regarding other primary or secondary endpoints and translational aims were not included in this acute toxicity analysis.
Trial Results
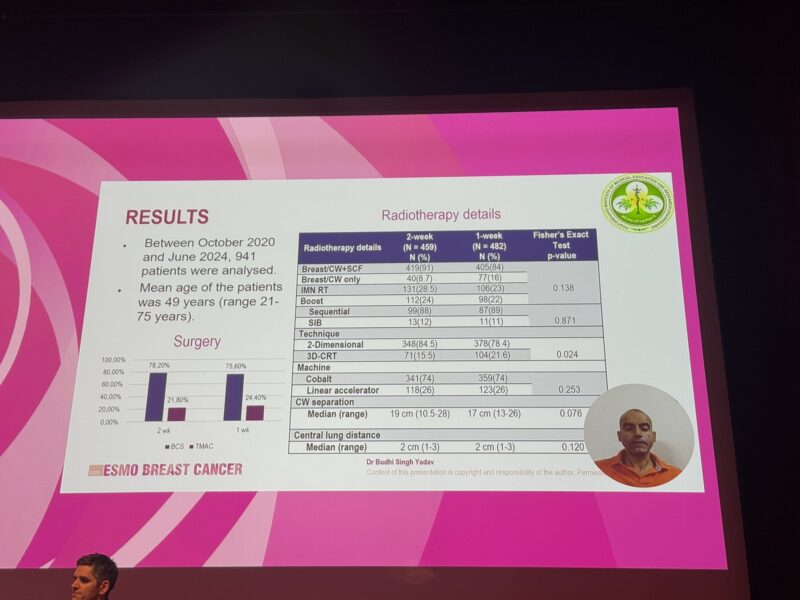
This analysis evaluated acute toxicity outcomes in 941 patients treated with either a 1-week (n=482) or a 2-week (n=459) radiotherapy schedule. Patient baseline characteristics were well-matched between the two treatment arms. The 1-week regimen delivered 26 Gy in 5 fractions, while the 2-week regimen provided 34 Gy in 10 fractions, with a boost dose administered to patients who had undergone breast-conserving surgery. Radiotherapy techniques utilized included 2D (78.4% vs 84.5%) and 3D-CRT (21.6% vs 15.5%).
Assessment of overall acute toxicity grades (scored 0-3) revealed comparable profiles for both schedules (p=0.295). Grade 0 toxicity occurred in 55% of the 1-week group vs 54% of the 2-week group, Grade 1 in 29.3% vs 33.8%, Grade 2 in 15.1% vs 11.8%, and Grade 3 in 0.6% vs 0.4%.
Focusing on specific toxicities, a statistically significant difference was observed for odynophagia, which was reported less often with the 1-week schedule (p=0.001). This difference was primarily seen in Grade 1 events (2.9% vs 8.9%) and the absence of symptoms (Grade 0: 95.6% vs 90%). Acute arm edema (Grade 1: 20.8% vs 17.6%; Grade 2: 4.1% vs 6.1%; p=0.227) and Grade 2 cough (1.9% vs 1%; p=0.869) rates were similar between the treatment arms.
source: Erika Hamilton/ X
Safety Profile (Toxicity)
The acute toxicity analysis indicates that both the 1-week and 2-week hypofractionated radiotherapy schedules demonstrated low rates of severe (Grade 3) toxicities, which were minimal with both approaches. While overall acute toxicity rates were similar between the two treatment durations, the study specifically found that odynophagia was experienced more frequently with the longer 2-week course compared to the shorter 1-week course. Acute arm edema and cough rates were comparable.
What This Means for Patients
These findings on acute toxicity from the HYPART trial are encouraging for patients requiring adjuvant radiotherapy for high-risk breast cancer. They suggest that a significantly shorter treatment schedule of just one week could potentially offer a similar, or even improved (in the case of odynophagia), acute toxicity profile compared to a two-week schedule. This could mean less time spent in treatment, potentially reducing the burden on patients without increasing immediate side effects. While these results focus on acute toxicity, the low rates of severe side effects in both arms are reassuring. Further results on long-term toxicity and efficacy endpoints will be crucial to fully understand the benefits of the 1-week schedule.