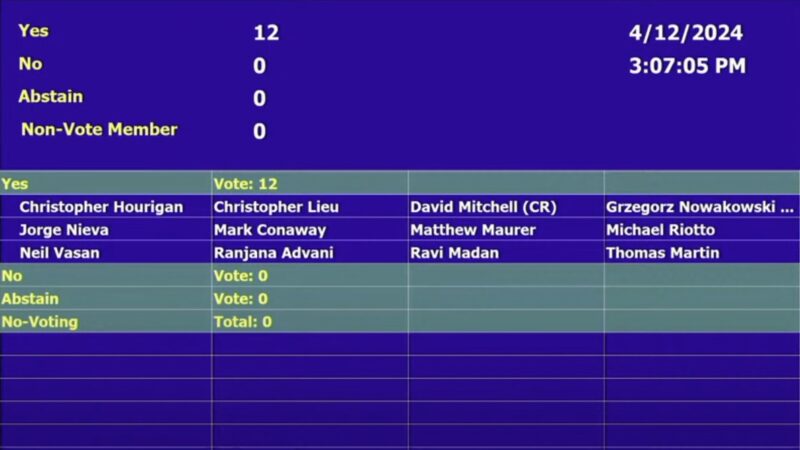
On April 12, 2024, FDA ODAC voted 12-0 in favor of using minimal residual disease (MRD) as an accelerated approval endpoint in multiple myeloma clinical trials. The OncoDaily Team provided a summary of important information and opinions from key opinion leaders about this significant milestone.
Carl Ola Landgren, Professor and the Director of Sylvester Myeloma Institute at the University of Miami, shared on LinkedIn:
“Great victory in myeloma today: ODAC voted 12-0 for MRD as an early endpoint for accelerated approval in multiple myeloma!
This will enable faster access to new, effective therapies for patients with multiple myeloma. There is an unmet medical need until we have a cure for multiple myeloma!
Honored to present the results from the EVIDENCE meta-analysis, work I pioneered 15 yrs ago. Congrats also to I2TEAMM led by IMF.”

Photo Carl Ola Landgren / LinkedIn
FDA ODAC votes 12-0 in favor of using MRD as an accelerated approval endpoint in myeloma clinical trials
Earlier today, the FDA Oncologic Drugs Advisory Committee (FDA ODAC) voted in favor of using minimal residual disease (MRD) as an accelerated approval endpoint in multiple myeloma clinical trials.
YouTube Broadcast of the April 12, 2024 Meeting: FDA ODAC Live Video
“Today’s ODAC positive vote (12-0 in favor) for MRD as an early endpoint for accelerated approval of new multiple myeloma drugs is fantastic news for patients diagnosed with multiple myeloma. With MRD as a new endpoint, it will give patients access to new therapies much faster! This is exactly what patients need and want,” said Dr. Carl Ola Landgren. “It is a milestone in the field of multiple myeloma and will set the stage for the development of novel therapies for other cancers.”
Oncology community celebrates this important milestone
“IMF celebrates ODAC’s historic decision on MRD testing as an early endpoint for accelerated myeloma drug approval. A decade of IMF research led by Dr. Durie and i2TEAMM; and University of Miami Sylvester Comprehensive Cancer Center contributed to this landmark decision.”, shared International Myeloma Foundation (IMF) on LinkedIn.

“The research and evidence presented by Dr. Landgren was impactful, positive, clear and convincing,” said Stephen D. Nimer, director of Sylvester Comprehensive Cancer Center. “The vote by the Oncologic Drugs Advisory Committee supporting the use of minimal residual disease to approve new multiple myeloma treatments will greatly speed up the development of new drugs for myeloma and help the thousands of patients with myeloma receive optimal therapy.”
Murali Janakiram, Associate Professor in the Department Hematology and Hematopoietic Cell Transplantation at City of Hope, shared on X (Twitter):
“ODAC Votes -12-0 to use MRD as an endpoint
ODAC team comments
- I have never before seen this effort
- Herculean effort
- 15 years
- blueprint for other biomarkers
- a precedent for other cancers
- raises the bar higher 🙂
-Hearty congrats to the i2TEAMM
-Thx Carl Ola Landgren, International Myeloma Foundation, Yelak Biru, Tom Martin, FDA Oncology, Vincent Rajkumar”.
“Kudos to the i2TEAMM led by Nikhil Munshi, Brian Durie and Jesus San Miguel for a monumental effort.”, wrote Vincent Rajkumar, Chairperson of the Board of Directors of International Myeloma Foundation and Professor at Mayo Clinic. “A great team effort involving numerous investigators, FDA Oncology, Pharma companies, patient advocates, non profit foundations, Myeloma Society.”
“Getting MRD as an endpoint was a great partnership between academia and industry and patient organization. A model to follow. Patients are three winner.”, shared on X (Twitter) Nikhil C. Munshi, Immediate Past President of Myeloma Society and Professor of Medicine at the Harvard Medical School.
Pashtoon Kasi, GI oncologist from Weill Cornell, added on his X (Twitter):
“Next: ctDNA MRD➖ as an endpoint for Colorectal Cancer.
Time to reconsider.
ctDNA➕📉DFS
ctDNA➖📈DFS”
About OncoDaily
OncoDaily was founded in 2023. It is a US-based oncology media platform, which features the latest news, insights, and patient stories from the world of oncology. Within short period of time it became one of the leading oncology media platforms globally.
OncoDaily gathers content from various sources, including social media posts from renowned oncologists from all over the world, news from oncology societies and cancer centers, patient and survivor stories, and career-related information for professionals.
The mission of OncoDaily is to empower patients, survivors, and professionals with the knowledge and inspiration they need to fight cancer. The motto of OncoDaily is “Cancer doesn’t take a day off – neither do we.”.


