On December 4, 2024, the U.S. Food and Drug Administration (FDA) approved durvalumab (Imfinzi, AstraZeneca) for adults with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
Durvalumab (brand name Imfinzi) is a monoclonal antibody developed by Medimmune, a part of AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody designed to block the interaction between programmed cell death ligand 1 (PD-L1) and PD-1 (CD279) receptors on T-cells. By inhibiting this interaction, durvalumab helps to activate the immune system to target and destroy cancer cells. It is a form of immune checkpoint inhibitor therapy, which has shown significant potential in treating various cancers by unmasking the immune system’s ability to fight malignancies.
Durvalumab is approved for the treatment of several types of cancer, including bladder, lung, and biliary tract cancers. It has shown efficacy in treating locally advanced or metastatic urothelial carcinoma that has either progressed during or after platinum-based chemotherapy or within twelve months of treatment. Additionally, it is approved for Stage III non-small cell lung cancer (NSCLC) in patients whose disease has not advanced following concurrent platinum-based chemotherapy and radiation therapy.
Durvalumab’s therapeutic applications also extend to small cell lung cancer (SCLC) and biliary tract cancer (BTC). It is used in combination with etoposide and either carboplatin or cisplatin as the first-line treatment for extensive-stage SCLC. In BTC, it is used in combination with gemcitabine and cisplatin for treating locally advanced or metastatic cases. These approvals reflect durvalumab’s broad applicability across several challenging cancers, providing patients with new options for treatment.
About the FDA Approval
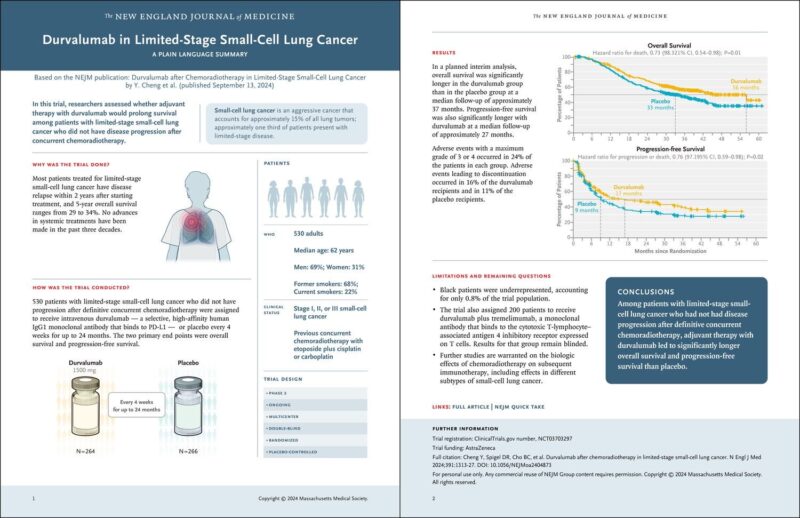
The approval study titled “Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer” published on The New England Journal of Medicine by ESMO was based on the results of the ADRIATIC trial, a randomized, double-blind, placebo-controlled study involving 730 patients with LS-SCLC. These patients had not experienced disease progression following the combination of chemotherapy and radiation. Participants were randomly assigned to receive either durvalumab as a single agent, durvalumab combined with tremelimumab, or a placebo.
Authors: Ying Cheng, David R. Spigel, Byoung Chul Cho, Konstantin K. Laktionov, Jian Fang, Yuanbin Chen, Yoshitaka Zenke, Ki Hyeong Lee, Qiming Wang, Alejandro Navarro, Reyes Bernabe, Eva Lotte Buchmeier, John Wen-Cheng Chang, Yoshimasa Shiraishi, Sema Sezgin Goksu, Andrzej Badzio, Anhui Shi, Davey B. Daniel, Nguyen Thi Thai Hoa, Milada Zemanova, Helen Mann, Hema Gowda, Haiyi Jiang, Suresh Senan, for the ADRIATIC Investigators

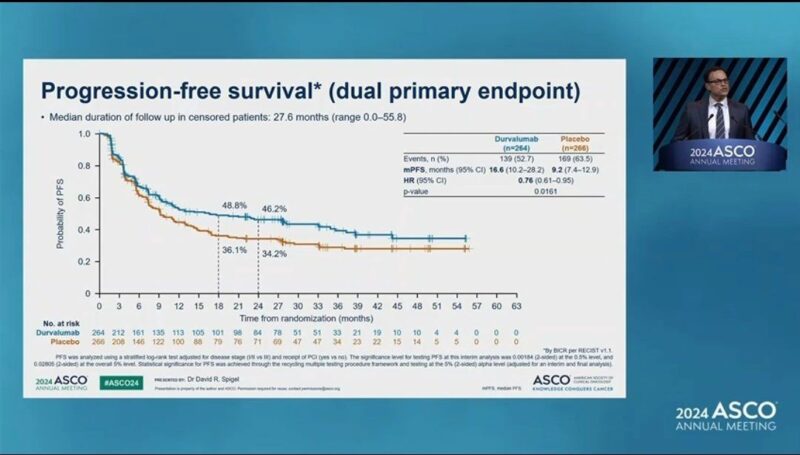
Key efficacy endpoints included overall survival (OS) and progression-free survival (PFS), which were assessed by blinded independent central review. Durvalumab showed a statistically significant improvement in OS compared to placebo, with a hazard ratio (HR) of 0.73 (95% CI: 0.57, 0.93; p-value 0.0104). The median OS was 55.9 months for the durvalumab group and 33.4 months for the placebo group. Additionally, durvalumab demonstrated a significant improvement in PFS compared to placebo, with a HR of 0.76 (95% CI: 0.61, 0.95; p-value 0.0161). The median PFS was 16.6 months for the durvalumab arm versus 9.2 months for the placebo arm.
The most common adverse reactions (occurring in ≥20% of patients) included pneumonitis or radiation pneumonitis and fatigue.
Dosing and Administration
The recommended dose of durvalumab for adults with LS-SCLC is 1,500 mg every 4 weeks for patients weighing ≥30 kg, and 20 mg/kg every 4 weeks for those weighing <30 kg, until disease progression, unacceptable toxicity, or a maximum duration of 24 months.
This approval is part of Project Orbis, an FDA Oncology Center of Excellence initiative aimed at streamlining the review process for oncology drugs in collaboration with international regulatory agencies. For this review, the FDA worked alongside the Australian Therapeutic Goods Administration (TGA), the Brazilian Health Regulatory Agency (ANVISA), Health Canada, and Switzerland’s Swissmedic (SMC). The European Medicines Agency (EMA) also observed the process, with ongoing reviews by other regulatory bodies.
Healthcare Professionals shared about this on Social Media:
“The durva approval for limited stage small cell lung cancer made my day I had a throughout conversation with my patient just two days ago, and now it’s been approved!”
“Breaking news in SCLC treatment!
Durvalumab is now FDA-approved for limited-stage SCLC based on the ADRIATIC trial. Adding 2 years of durvalumab after chemoradiation improves both PFS and OS (HR 0.73).
Note: Avoid concurrent use with chemoradiation.
This approval marks an important step forward for patients with limited-stage SCLC!”
“And it gets approved as expected.
Durvalumab gets approval in limited stage SCLC post ctrt stable disease based upon Adriatic trial.
It will be interesting to see what these pts get on progression post maintenance durvalumab in real world.”