Eytan Ruppin, Chief of the Cancer Data Science Laboratory, shared a paper he and his colleagues authored on X:
“Delighted to announce the publication of our 10 hallmarks of AI in precision oncology Review paper in Nature Cancer! This work provides a comprehensive, up-to-date synthesis of AI’s transformative role in cancer care. Check it out here.
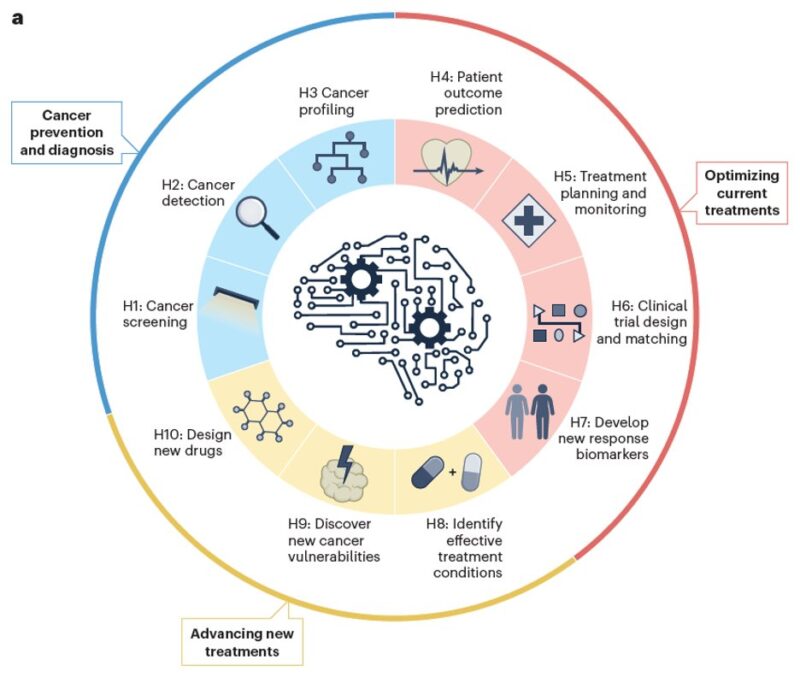
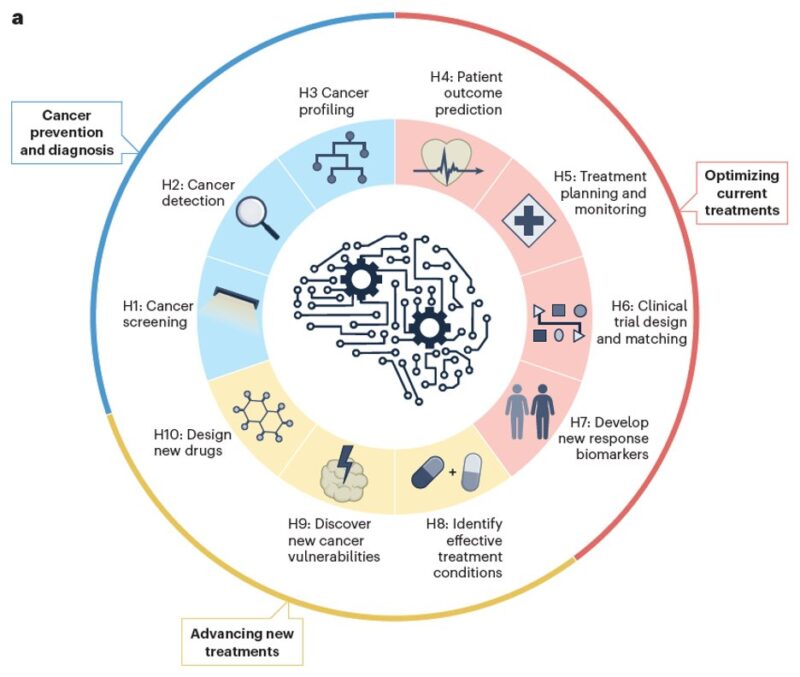
Cancer poses a profound global health challenge. As biomedical data expands, so does AI’s potential. In this review, we summarize hallmarks of AI is driving precision oncology, spanning prevention, diagnosis, treatment, and drug discovery.
We begin by examining major AI models and data modalities in precision oncology, covering machine learning, deep learning, transformers, and large language models. We also discuss diverse data types, including medical imaging, clinical records, and omics data.
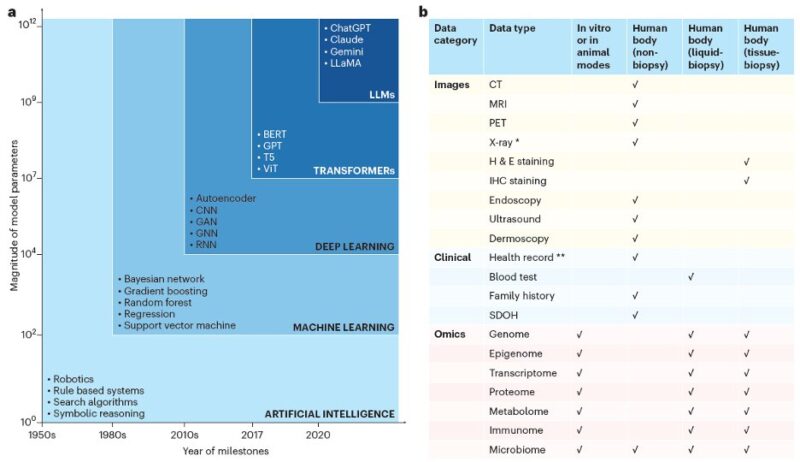
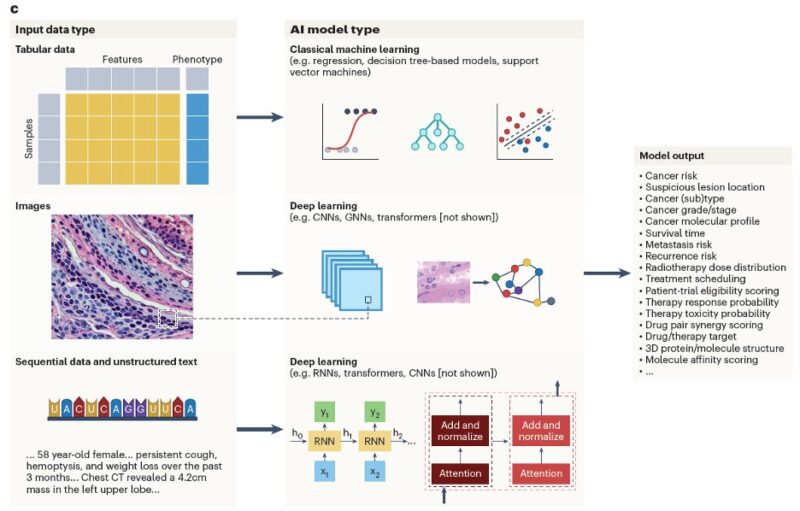
While numerous AI models exist, selecting the right one depends on data type and research goals. Aligning model architecture with these factors is crucial for optimizing AI’s impact in cancer care. e.g.:
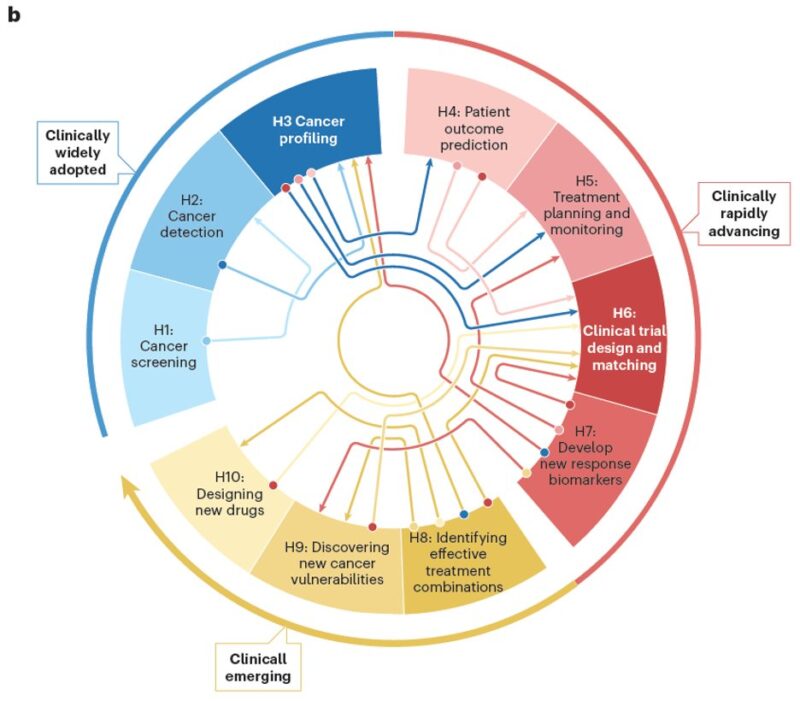
Then, we classify recent advances in AI oncology into three categories, each encompassing a set of carefully defined hallmarks illustrating AI’s impact – from early detection to personalized therapies and innovative drug development.
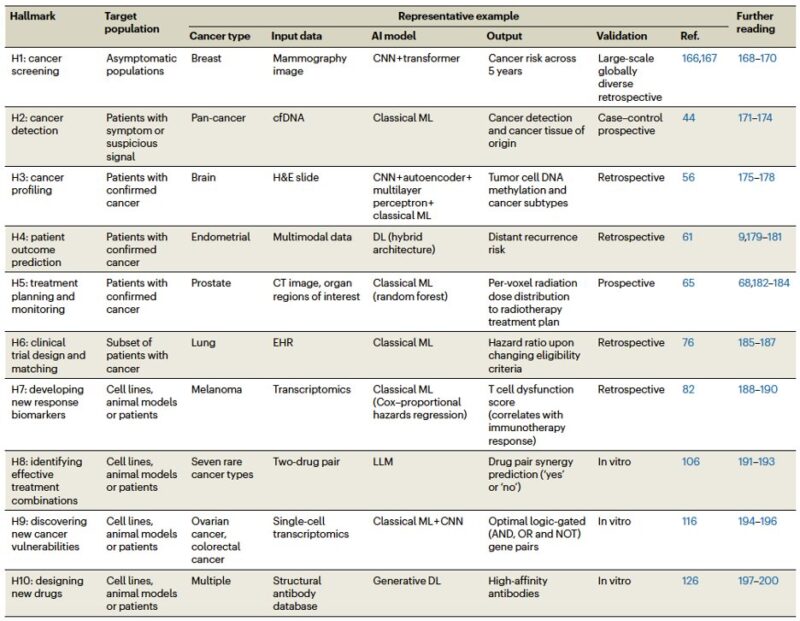
To aid non-experts, our review provides a concrete example for each hallmark. For every case, we detail the cancer type, input data, AI model, output, and validation method, and we also include a list of relevant reviews for further reading.
A key takeaway: These hallmarks do not function in isolation – they form an interconnected ecosystem, collaboratively shaping the future of cancer care.
Our review also analyzes AI applications in interventional clinical trials, revealing that AI remains underutilized in influencing trial design and data analysis.
What are the biggest barriers to AI’s clinical translation? We identify four key challenges: data quality and quantity, model accuracy, clinical relevance, and demonstrable patient benefit. We further propose actionable solutions to address these challenges.
Some guiding principles for clinical AI Fairness: Use high-quality, diverse datasets Interpretability: Avoid black-box models without efforts toward explainability Relevance: Use clinical input features and performance measures Equability: Complex models are not always better.
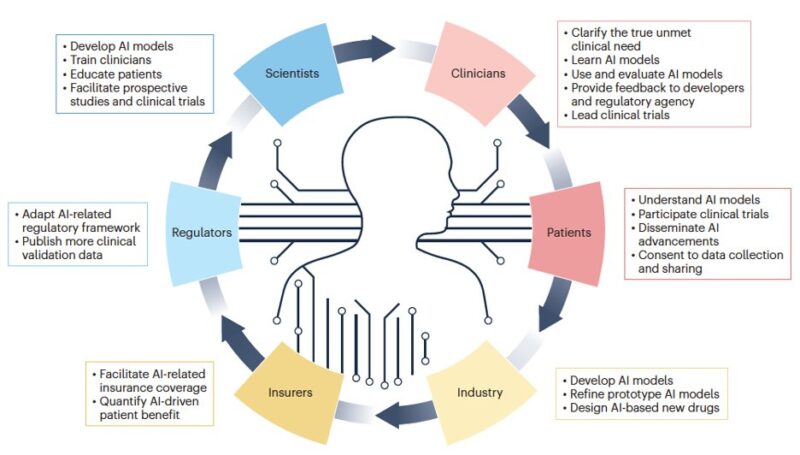
Ultimately, realizing AI’s clinical potential requires collaboration among scientists, clinicians, patients, industry, insurers, and regulatory bodies to enable seamless integration into routine oncology practice.
We hope our review provides a comprehensive understanding of AI’s role in precision oncology, fostering informed discussions on its potential to reduce cancer mortality and transform cancer care.
A huge thank you to our co-authors Tiangen Chang, Seongyong Park, Alejandro Schäffer, Peng Jiang, and to the experts who provided valuable feedback for shaping this work – Drs. Sheila Rajagopal and Ze’ev Ronai, and many more!
We encourage you to read, share, and join the discussion as we collectively harness AI to push the boundaries of cancer care. Read the full paper.”
Hallmarks of artificial intelligence contributions to precision oncology
Authors: Tian-Gen Chang et al.



